4127
3D PRF Thermometry of the Liver
Waqas Majeed1, Sunil Patil1, Pan Su1, Rainer Schneider2, Henrik Odéen3, Dennis L. Parker3, John Roberts3, Jiachen Zhuo4, and Himanshu Bhat1
1Siemens Medical Solutions USA Inc., Malvern, PA, United States, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Department of Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States, 4Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States
1Siemens Medical Solutions USA Inc., Malvern, PA, United States, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Department of Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States, 4Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States
Synopsis
Anatomical misregistration and background phase variation due to physiological motion are two major sources of inaccuracy in PRF thermometry of the liver. We propose a pipeline consisting of 3D segmented EPI acquisition, 3D deformable motion correction and PCA-based background phase correction for liver thermometry. 3D acquisition results in an increase in SNR and makes 3D registration possible. PCA-based background phase correction improves ΔT quality over a more commonly used dictionary-based method. The proposed pipeline was used to achieve 75th percentile ΔT bias and standard deviation values of 1.70°C and 1.65°C respectively over whole-liver in one healthy volunteer.
Introduction
Microwave ablation is a novel way of treating liver cancer lesions1. Usually these procedures are performed under CT guidance where the interventional radiologist has no thermal feedback regarding heating in the tumor. Proton Resonance Frequency (PRF) thermometry is a widely used MRI based technique to monitor changes in tissue temperature in response to thermal therapy2. However, motion related anatomical misregistration and background phase variation are two of the key factors limiting clinical application of PRF thermometry to guide microwave ablation procedures in the liver3. In this abstract, we present a novel acquisition and processing pipeline for liver thermometry to overcome these challenges. We propose the use of segmented 3D Echo Planar Imaging (EPI) to permit 3D deformable registration, and to improve signal to noise ratio (SNR) and spatial coverage. Principle component analysis (PCA) based baseline correction is used to remove baseline phase variation.Methods
Data Acquisition: All human imaging protocols were approved by local Institutional Review Board. One healthy volunteer was imaged on a 3T scanner (MAGNETOM Prisma fit, Siemens Healthcare, Erlangen, Germany) using a prototype 3D segmented EPI sequence4 modified to incorporate interactive pause capability. The following parameters were used: TR 24ms, TE 5.1ms, fat saturation, 2x3x3mm3 spatial resolution, 192x105x10 matrix with 20% slice oversampling, EPI factor 9, in-plane acceleration R = 2, 100 repetitions, 1.75 seconds per 3D volume. Interactive pause functionality was used to divide the acquisition into ~16-20s breath-holds.Preprocessing: Each image was registered to the first image in the series using a deformable 3D registration algorithm5. First image of each breath-hold was discarded to avoid transient magnetization effects. First 40 images of the resultant series were chosen as baseline images (referred to as baseline series). The remaining images (referred to as therm series) were used to compute temperature difference maps.
Background phase removal and ΔT estimation: We utilized a PCA based algorithm to remove motion related phase changes6 : Complex mean of the baseline images BAve was obtained. PCA was performed over a liver mask on the series consisting of unwrapped complex phase difference between baseline images and BAve. First 20 of the resultant eigen-images were retained as basis images for correcting the therm series. Complex phase difference was computed between each therm image and the corresponding previous therm image. BAve was used as the image before the first therm image. Baseline correction was performed by removing the projection of each unwrapped difference image onto the subspace spanned by the basis images. Temporal cumulative sum of the resultant series was obtained.
For comparison, we also performed baseline correction with the Dictionary-based approach, where the best matching baseline image is looked up for each therm image7. For both approaches, average phase over the mask was removed after baseline correction to eliminate global phase drift. Resultant phase difference images were scaled by -1/(γB0xTE×0.01ppm/°C) to estimate temperature difference relative to the first acquisition. Since the expected temperature change was 0 in absence of external heating, we used temporal mean (μT) and standard deviation (σT) of the estimated ΔT series to assess estimation bias and variability.
Results
3D deformable registration adequately corrects inter-breathhold motion, as evidenced by a reduction in temporal standard deviation of magnitude images (Figure 1). Thermometry maps obtained using PCA based approach exhibit excellent precision and accuracy (Figure 2). |μT| and σT for PRF measurements corrected using the dictionary-based are greater than those corrected with the PCA-based approach (Figure 2, 3 and 4).Discussion
Our results suggest that PRF measurements acquired and processed using the proposed pipeline exhibit excellent precision and accuracy. |μT| and σT are much smaller in most of the liver than the threshold for irreversible tissue damage3, suggesting the feasibility of PRF based guidance for liver ablation. Consistent with results reported by Majeed et al6, the PCA based approach outperforms the existing dictionary-based approach.To our knowledge, all liver thermometry studies to date have utilized 2D acquisition schemes. Although these studies rely on navigators, respiratory triggering and breath-holds to reduce the impact of respiratory motion, residual inter-scan motion cannot be avoided. Limited spatial coverage in slice direction makes 3D registration unfeasible, resulting in compromised anatomical precision. The proposed segmented EPI based approach overcomes this challenge while keeping the frame rate within reasonable limits. Additionally, 3D acquisition results in higher SNR for a given resolution and spatial coverage.
We have chosen to use TE = 5.1ms, which is much smaller than the T2* of the normal liver at 3T (14.5±6.7ms)8. Although TE = T2* is the optimal choice of TE for PRF thermometry8, TE values smaller than T2* of the normal tissue may be required in practice to avoid intravoxel dephasing due to B0 inhomogeneity induced by the ablation device. 3D acquisition may prove crucial to counteract the resulting loss of temperature sensitivity and SNR.
The interactive pause functionality described in this study enables multi-breathhold acquisitions without the need to stop the scan. Additionally, it can be used to pause the acquisition during microwave-based ablation to avoid unnecessary acquisition of noise corrupted images.
Future work will focus on evaluation on a larger sample, further protocol optimization and comparison with additional existing approaches.
Acknowledgements
No acknowledgement found.References
- Simon, Caroline J., Damian E. Dupuy, and William W. Mayo-Smith. "Microwave ablation: principles and applications." Radiographics 25.suppl_1 (2005): S69-S83.
- Rieke, Viola, and Kim Butts Pauly. "MR thermometry." Journal of Magnetic Resonance Imaging 27.2 (2008): 376-390.
- Kägebein, Urte, et al. "Motion correction in proton resonance frequency–based thermometry in the liver." Topics in Magnetic Resonance Imaging 27.1 (2018): 53-61.
- Odéen, Henrik, et al. "Sampling strategies for subsampled segmented EPI PRF thermometry in MR guided high intensity focused ultrasound." Medical physics 41.9 (2014).
- Fieseler, Michael, et al. "Motion estimation in PET-MRI based on dual registration: preliminary results for human data." EJNMMI physics 1. Suppl 1 (2014).
- Majeed, Waqas, et al. “A Principal Component Analysis based Multi-baseline Phase Correction Method for PRF Thermometry.” Proceedings of ISMRM 27th Annual Meeting & Exhibition (2019): 3818.
- Roujol, Sébastien, et al. "Real‐time MR‐thermometry and dosimetry for interventional guidance on abdominal organs." Magnetic Resonance in Medicine 63.4 (2010): 1080-1087.
- Holbrook, Andrew B., et al. "Real‐time MR thermometry for monitoring HIFU ablations of the liver." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 63.2 (2010): 365-373.
Figures
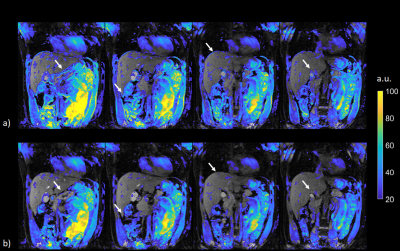
Figure 1:Temporal standard deviation of magnitude image series. Magnitude standard deviation maps without and with motion
correction are displayed in a) and b) respectively. Standard deviation values above 20 are
overlayed on the first magnitude image in the series. Four out of 10 slices are
displayed. Motion correction results in a reduction in magnitude standard
deviation especially in the edge voxels, as indicated by arrows.
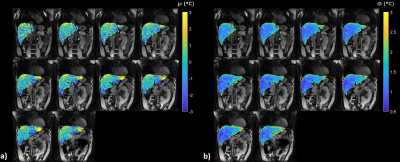
Figure 2: μT and σT with PCA based baseline
correction. μT and σT are displayed
for all 10 slices. Third quartile values of |μT| and σT over a whole-liver ROI are 1.70°C and 1.65°C respectively, suggesting excellent
precision and accuracy for majority of the liver voxels.
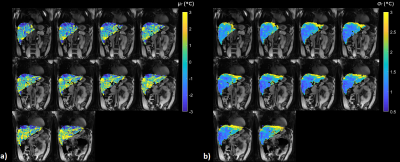
Figure 3: μT and σT with
dictionary-based baseline correction. Third
quartile values of |μT| and σT over a whole-liver ROI are 2.71 °C and 1.91 °C respectively.
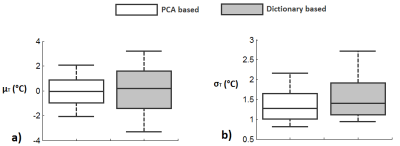
Figure 4: μT and σT over a whole-liver ROI. Box plots for μT and σT obtained
from a whole-liver ROI are shown in a) and b). PCA-based approach results in a
reduction in |μT| and σT as compared
with the dictionary-based approach.