4120
Improving in situ acoustic intensity estimation by augmenting MR acoustic radiation force imaging with MR elastography1Electrical Engineering, Stanford University, Stanford, CA, United States, 2Radiology, Stanford University, Stanford, CA, United States
Synopsis
MR acoustic radiation force imaging (MR-ARFI) can be used to localize the focal spot for non-thermal transcranial ultrasound therapies. The acoustic radiation force is proportional to the applied acoustic intensity, meaning that tissue displacements measured with MR-ARFI can potentially be used to estimate the acoustic intensity at the target. However, variable brain stiffness is an obstacle to obtaining accurate acoustic intensity estimates. Using gelatin phantoms with varying stiffnesses, we demonstrate that stiffness information from MR elastography can be used in combination with MR-ARFI to improve in situ estimates of acoustic intensity. This could enable safer and more effective treatments.
Introduction
The skull presents a significant challenge to focused ultrasound brain therapies. It attenuates the acoustic intensity at the focal spot, and there can be up to a four-fold variation due to skull heterogeneity across patients1. This is problematic for transcranial therapies, such as blood-brain barrier opening, where modest increases in acoustic intensity can cause tissue damage through inertial cavitation2. MR acoustic radiation force imaging (MR-ARFI) methods have been developed for in vivo focal spot localization3, and they have been applied successfully to procedures such as neuromodulation4 and blood-brain barrier opening5.In addition to localization, MR-ARFI has the potential to estimate the in situ acoustic intensity6, since the acoustic radiation force is proportional to the acoustic intensity at the focus7. However, determination of acoustic intensity from MR-ARFI measurements of tissue displacement requires knowledge of the tissue’s mechanical properties, which vary across patients, brain regions8, and disease states such as Alzheimer’s9. Applying the same ultrasound pulse to two patients: one with a thin skull (high transmitted acoustic intensity) but a stiff brain and another with a thick skull (low transmitted acoustic intensity) but a soft brain, might result in the same tissue displacement even though the acoustic intensity at the focus is different. In this study, we investigate whether stiffness information from MR elastography (MRE) can be used to correct for this bias and improve in situ MR-ARFI-based estimates of acoustic intensity.
Methods
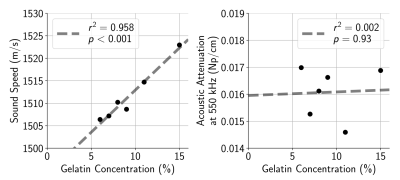
Phantoms were fabricated using 100-bloom gelatin (Nitta Gelatin, Morrisville, NC) dissolved in a suspension of 50% deionized water and 50% evaporated milk10. Phantom stiffness was adjusted by varying gelatin concentration. To account for the influence of acoustic properties on the applied acoustic radiation force7, the sound speed and acoustic attenuation at 550 kHz was measured for each phantom using a through-transmission method10 with a single-element planar transducer and hydrophone (ONDA, Sunnyvale, CA).MR-ARFI data were acquired at 3T (Signa Excite, GE Healthcare, Milwaukee, WI) using a 2DFT spin-echo sequence (20 cm × 20 cm field of view, 0.7 mm slice thickness, 256×128 acquisition matrix, 39 ms echo time, 500 ms repetition time) with repeated bipolar gradients and 16 ms ultrasound pulse length11. Complex phase difference images of MR-ARFI data acquired with alternating motion encoding gradient polarities were scaled to obtain displacement maps. Sonications were performed using applied electric powers ranging from 50 W to 200 W at 550 kHz (ExAblate 2100, Insightec Ltd., Haifa, Israel) with a 75 mm focal depth. Applied electric powers corresponded to spatial-peak pulse-average acoustic intensities varying from 200 W/cm2 to 900 W/cm2 as measured in free water with a fiber-optic hydrophone (Precision Acoustics, Dorset, UK), and they were de-rated using acoustic attenuation and path lengths measured from each phantom to acquire in situ acoustic intensity estimates.
MRE was performed using a single-shot, spin-echo, echo-planar imaging pulse sequence12. Shear waves were induced in the phantom by coupling it to a passive driver with a 60 Hz actuation frequency, and wave images (128×128 acquisition matrix, 22 cm × 22 cm field of view, 3.5 mm slice thickness, 2× acceleration factor, 58.6 ms echo time, 2000 ms repetition time) were acquired with sixteen phase offsets sampled over each period of motion. Sinusoidal motion-encoding gradients were applied in the ±x, ±y, and ±z directions, and complex shear modulus maps were reconstructed using a 3D direct inversion algorithm12. The mean magnitude of the complex shear modulus at the focal spot was recorded for each phantom.
Results
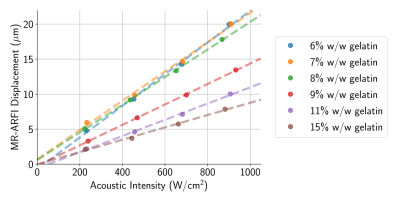
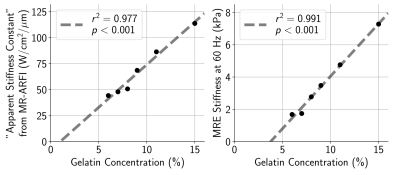
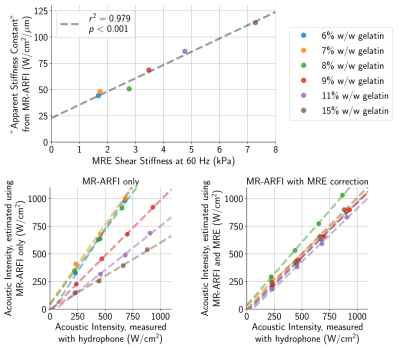
When sonicated using approximately equal acoustic intensities, there was immense variability (2-3×) in MR-ARFI displacements across phantoms with different gelatin concentrations (Fig. 1). Though phantoms were found to have slightly dissimilar acoustic properties (Fig. 2), meaning the applied acoustic radiation force differed across phantoms for the same applied acoustic intensity, it was not enough to solely explain the extensive discrepancy in MR-ARFI displacements (Fig. 1).The inverse slope (W/cm2/μm) of each MR-ARFI displacement vs. acoustic intensity line (from Fig. 1), was used as a MR-ARFI-derived stiffness metric, or “apparent stiffness constant”. Fig. 3 shows that the phantom’s gelatin concentration was highly correlated with stiffness estimated with both MR-ARFI and MRE. The “apparent stiffness constant” was strongly correlated with MRE stiffness measurements (Fig. 4, top), and scaling each phantom’s measured MR-ARFI displacements by their estimated MRE stiffness led to superior agreement between estimated acoustic intensities across all the phantoms (Fig. 4, bottom right).
Discussion
This simplistic correction scheme used in this study assumed that the phantoms were purely elastic and did not account for the dampening effects of viscosity on the measured MR-ARFI displacements13. More complex mechanical models are necessary for better representing the viscoelastic nature of brain tissue. Nonetheless, the results of this study emphasize the importance of understanding the tissue’s viscoelastic properties when attempting to estimate the acoustic intensity delivered to the treatment target. Our results demonstrate the potential for MR-ARFI to provide both focal spot localization and estimated acoustic intensity in combination with MRE stiffness estimates, which would be crucial for enhancing the safety and efficacy of focused ultrasound therapies. Future studies will focus on optimizing MR-ARFI and MRE parameters as well as investigating mechanical models that account for viscoelasticity.Acknowledgements
This work was supported by NIH T32 CA009695, T32 EB009653, and NSF DGE 1656518. The authors would like to acknowledge Richard L. Ehman and Kevin Glaser from the Mayo Clinic for providing the MR elastography sequence and actuator device. We would also like to thank Patricia Lan for her help acquiring MR elastography images.References
1. Vyas U., et al. Predicting variation in subject thermal response during transcranial magnetic resonance guided focused ultrasound surgery: Comparison in seventeen subject datasets. Medical Physics. 2016;43(9):5170-80.
2. Tung Y. et al. In vivo transcranial cavitation threshold detection during ultrasound-induced blood–brain barrier opening in mice. Physics in Medicine & Biology. 2010;55(20):6141.
3. McDannold N. et al. Magnetic resonance acoustic radiation force imaging. Medical Physics. 2008;35(8):3748-58.
4. Gaur P., et al. In vivo MR-ARFI for transcranial focused ultrasound in large animals. In 27th International Society for Magnetic Resonance in Medicine Annual Meeting, Paris. 2018.
5. Magnin R., et al. Magnetic resonance-guided motorized transcranial ultrasound system for blood-brain barrier permeabilization along arbitrary trajectories in rodents. Journal of Therapeutic Ultrasound. 2015;3(1):22.
6. Gerstenmayer M., et al. Magnetic resonance acoustic radiation force imaging for in vivo estimation of ultrasonic transmission factor through rat skulls. In 16th International Symposium on Therapeutic Ultrasound, Tel Aviv. 2016; p. 372-375.
7. Nightingale K., et al. Acoustic radiation force impulse imaging: in vivo demonstration of clinical feasibility. Ultrasound in Medicine & Biology. 2002;28(2):227-35.
8. Arani A., et al. Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. NeuroImage. 2015;111:59-64.
9. Murphy M., et al. Regional brain stiffness changes across the Alzheimer's disease spectrum. NeuroImage: Clinical. 2016;10:283-90.
10. Farrer, A. I., et al. Characterization and evaluation of tissue-mimicking gelatin phantoms for use with MRgFUS. Journal of Therapeutic Ultrasound, 2015 Dec;3(1):9.
11. Bitton R., et al. Toward MR-guided high intensity focused ultrasound for presurgical localization: Focused ultrasound lesions in cadaveric breast tissue. JMRI 2012;35:1089–1097.
12. Pepin K., et al. MR elastography analysis of glioma stiffness and IDH1-mutation status. American Journal of Neuroradiology. 2018;39(1):31-6.
13. Bercoff J. et al. The role of viscosity in the impulse diffraction field of elastic waves induced by the acoustic radiation force. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 2004;51(11):1523-36.
Figures