4119
Selective fast focal switching of high-contrast-ratio magnetocaloric MRI labels with focused ultrasound
G. Wilson Miller1,2, Yekaterina Gilbo2, Stephen Dodd3, Alan P Koretsky3, Hatem ElBidweihy4, and Mladen Barbic5
1Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, United States, 2Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 3National Institutes of Health, Bethesda, MD, United States, 4United States Naval Academy, Annapolis, MD, United States, 5HHMI - Janelia Research Campus, Ashburn, VA, United States
1Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, United States, 2Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 3National Institutes of Health, Bethesda, MD, United States, 4United States Naval Academy, Annapolis, MD, United States, 5HHMI - Janelia Research Campus, Ashburn, VA, United States
Synopsis
We describe the use of MRI-compatible focused ultrasound (FUS) for selective fast focal switching of high-contrast-ratio magnetocaloric MRI particle labels. Lanthanum-Iron-Silicon (La-Fe-Si) particles that have sharp first-order magnetic phase transitions at physiological temperatures and MRI field strengths were used as MRI labels. Non-invasive rapid thermal switching of these magnetocaloric MRI particle labels with focused ultrasound was clearly demonstrated at physiological temperatures within a clinical 1.5T MR scanner. We have thereby shown that high differential contrast ratio magnetocaloric MRI labels can be switched non-invasively, rapidly, and selectively under conditions relevant to clinical MRI.
Introduction
Magnetocaloric materials previously developed for magnetic refrigeration1,2, data storage3, and spintronics applications4 were recently proposed as novel high-contrast labels for MRI5-7. Proof-of-concept MRI experiments have shown that the sharp first-order magnetic phase transitions of these materials at physiological temperatures and Tesla-scale DC magnetic field values provide an ideal match to the requirements for switchable MRI labels5-7. A drawback of prior experiments was the necessity of thermally cycling the entire phantom at slow ramp rates to induce the embedded labels’ magnetic phase transitions. Here, we explore the potential of using focused ultrasound (FUS)8-11 to locally, selectively, and rapidly thermally switch Lanthanum-Iron-Silicon (La-Fe-Si)12-14 MRI particle labels at physiological temperatures in a 1.5 Tesla magnetic field.Methods
A Lanthanum-Iron-Silicon (La-Fe-Si) powder sample (100 μm-250 μm particle size) was obtained from a commercial vendor (Calorivac H product line from Vacuumschmelze). Temperature dependent magnetic properties of the powder sample were measured in a vibrating sample magnetometer (VSM) (Versalab System from Quantum Design, Inc.) at 1.5T, to match the operating field of a 1.5T MR scanner (Siemens Avanto). For the MRI characterization, individual La-Fe-Si particles were placed ~15 mm apart, sandwiched between two layers of Zerdine hydrogel phantom material. An MRI-compatible focused ultrasound system10 (FUS Instruments) was used for focused delivery of ultrasonic energy (35W for 20s, 1.1 MHz continuous-wave) to individual particles embedded in the phantom, while MR imaging was simultaneously performed to monitor image contrast. A spoiled gradient-echo pulse sequence (TR/TE/FA = 39 ms/2.4 ms/20°; voxel size = 1 mm×1 mm; FOV = 128 mm×128 mm; readout bandwidth = 1185 Hz/pixel; 5 s per image) was used to acquire a time series of 24 consecutive images of a single 3 mm slice at the location of the particles during the time period spanning each 20s sonication. Due to rapid dephasing of the MR signal in the vicinity of each particle, it was not possible to use MR thermometry to monitor FUS-induced temperature changes during thermal switching. Instead, the magnitude and time course of FUS-induced heating were quantified separately by sonicating a spot away from the particles while measuring the proton resonance frequency shift8 using the same imaging pulse sequence but with longer TE (5 ms).Results
Figure 1 shows the VSM measurements of the magnetic moment of La-Fe-Si powder sample as a function of temperature in the bias DC magnetic field of 1.5T. The sample exhibits a sharp magnetic transition with narrow hysteresis over a narrow range of physiologically relevant temperatures (~37°C = 310 K). Figure 2 shows a sequence of representative gradient-echo images, illustrating the effect of sequential delivery of focused ultrasound energy to individual La-Fe-Si particles in the phantom. Rapid and selective magnetocaloric particle MRI label switching is evident in the time course images. Figure 3 shows MR thermometry results, including peak temperature evolution at the hottest pixel (red curve) and mean temperature over surrounding pixels (blue curve). Comparison with the time-course images in Figure 2 indicates that the magnetocaloric particles switch “off” at approximately 15-20° above the room temperature of ~21°, which corresponds to a range slightly above body temperature.Discussion and Conclusion
Non-invasive and selective fast focal switching of high-contrast-ratio magnetocaloric La-Fe-Si particle MRI labels was demonstrated at physiological temperatures and MRI field strengths using focused ultrasound. Development of novel contrast mechanisms and agents for MRI is critical for further advancements in label readout of physiological conditions, non-invasive cell imaging, and tracking in vivo15-19. Our results provide further impetus for the development of high differential contrast ratio switchable MRI labels using magnetocaloric materials in microparticle form and establish a practical avenue for non-invasive activation. Future work will include preparing such materials for multi-functional imaging by tuning their physical properties through materials science techniques as well as developing MRI-compatible instrumentation for controlled switching of these materials in vivo.Acknowledgements
This research was supported by the Howard Hughes Medical Institute, United States Naval Academy, University of Virginia Focused Ultrasound Center, and the NINDS Intramural Research Program of the National Institutes of Health. We thank Brian Bell and Kevin Ackerman of Vacuumschmelze company for generously providing us with the Calorivac H samples of La-Fe-Si powder.References
[1] V. Franco, J. S. Blazquez, B. Ingale, A. Conde, The Magnetocaloric Effect and Magnetic Refrigeration Near Room Temperature: Materials and Models. Annu Rev Mater Res 42 305 (2012). [2] X. Moya, S. Kar-Narayan, M. D. Mathur. Caloric materials near ferroic phase transitions. Nature Materials 13 439 (2014). [3] J. U. Thiele, S. Maat, and E. E. Fullerton, FeRh/FePt exchange spring films for thermally assisted magnetic recording media, Appl. Phys. Lett. 82, 2859 (2003). [4] X. Marti, I. Fina, C. Frontera, J. Liu, P. Wadley, Q. He, et al. Room-temperature antiferromagnetic memory resistor. Nature Materials 13, 367 (2014). [5] M. Barbic, T. D. Harris, S. Dodd, H. D. Morris, A. P. Koretsky, B. Marcheschi, A. Huston, and N. R. Dilley, Magneto-Caloric Materials as Tunable and Switchable Labels for MRI, Proc. Intl. Soc. Mag. Reson. Med. 25 (2017) 0999, 25th Annual Meeting of ISMRM; 2017; Honolulu, HI, USA. [6] M. Barbic, S. Dodd, H. D. Morris, N. R. Dilley, B. Marcheschi, A. Huston, T. D. Harris, and A. P. Koretsky, “Magneto-Caloric Materials as Switchable High Differential Contrast MRI Labels” Magnetic Resonance in Medicine 81, 2238 (2019). [7] M. Barbic, H. ElBidweihy, S. Dodd, H. D. Morris, and A. P. Koretsky, “Magneto-Caloric Materials as Symmetric Reverse-Contrast Switchable MRI Labels” Proc. Intl. Soc. Mag. Reson. Med. 27, (2019) 3977, 27th Annual Meeting of ISMRM; 2019; Montreal, Canada. [8] Cline HE, Hynynen K, Hardy CJ, Watkins RD, Schenck JF, Jolesz FA. MR temperature mapping of focused ultrasound surgery. Magn Reson Med. 1994;31(6):628-36. [9] Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5(4):321-7. [10] Chopra R, Curiel L, Staruch R, Morrison L, Hynynen K. An MRI-compatible system for focused ultrasound experiments in small animal models. Med Phys. 2009;36(5):1867-74. [11] Jolesz FA. MRI-guided focused ultrasound surgery. Annu Rev Med. 2009; 60:417-30. [12] Fujita A, Fujieda S, Hasegawa Y, Fukamichi K. Itinerant-electron metamagnetic transition and large magnetocaloric effects in La(FexSi1-x)(13) compounds and their hydrides. Phys Rev B. 2003; 67(10). [13] M. Katter, V. Zellmann, G. W. Reppel, and K. Uestuener, Magnetocaloric Properties of La(Fe,Co,Si)13, Bulk Material Prepared by Powder Metallurgy, IEEE Trans. Magn. 44, 3044 (2008). [14] B. R. Hansen, L. T. Kuhn, C. R. H. Bahl, M. Lundberg, C. Ancona-Torres, M. Katter, Properties of magnetocaloric La(Fe,Co,Si)13 produced by powder metallurgy, Journal of Magnetism and Magnetic Materials 322, 3447 (2010). [15] E. M. Shapiro, S. Skrtic, K. Sharer, J. M. Hill, C. E. Dunbar, and A. P. Koretsky, MRI detection of single particles for cellular imaging, Proc. Natl. Acad. Sci. U S A. 101, 10901 (2004). [16] J. W. M. Bulte, D. L. Kraitchman, Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 17,484 (2004). [17] G. Zabow, S. Dodd, J. Moreland, and A. P. Koretsky, Micro-engineered local field control for high-sensitivity multispectral MRI, Nature 453, 1058 (2008). [18] E. T. Ahrens and J. W. M. Bulte, Tracking immune cells in vivo using magnetic resonance imaging, Nature Reviews Immunology, 13, 755 (2013). [19] G. Zabow, S. Dodd, and A. P. Koretsky, Shape-changing magnetic assemblies as high-sensitivity NMR-readable nanoprobes, Nature 520, 73 (2015).Figures
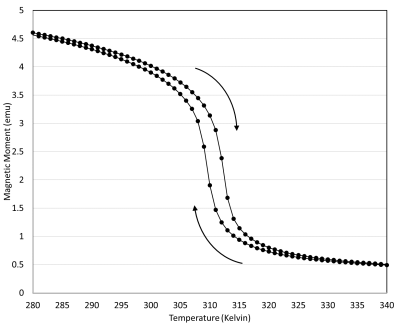
Figure 1: Vibrating
sample magnetometry (VSM) measurement of Magnetic Moment vs. Temperature of
Lanthanum-Iron-Silicon (La-Fe-Si) powder sample at 1.5T. The material exhibits a
sharp negative-slope first-order magnetic phase transition in the bias DC
magnetic field of 1.5 Tesla at physiological temperatures (~37°C = 310 K).
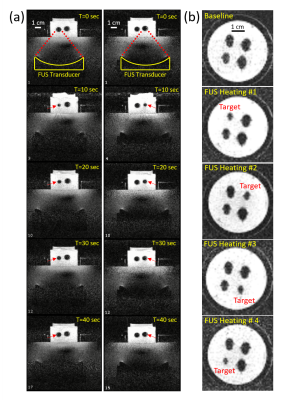
Figure 2: (a) A
sequence of gradient-echo images acquired in a vertical plane through two ~100 μm La-Fe-Si particles, while the two particles
are sequentially heated by FUS within the 1.5T
MRI scanner. The signal void around each particle decreases in size as
the temperature increases and returns to baseline upon cooling. The FUS transducer,
positioned sequentially under each magnetocaloric particle, is clearly visible
at the bottom of each image. (b) A
sequence of images acquired in a horizontal plane through all 4 particles embedded in
the phantom, as each particle is heated in turn.
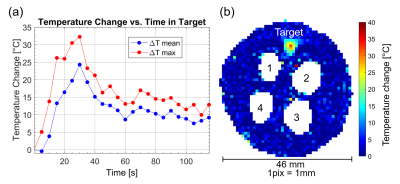
Figure 3: MR
thermometry of focused ultrasound heating. (a) Temperature evolution within a 3 mm x 3 mm target region of the gel
phantom away from the magnetocaloric particle locations, spanning a 20s
continuous-wave test sonication at 35W. (b) Temperature map showing maximum
temperature rise during the test sonication. Regions with low MR signal
intensity are masked, including those regions surrounding the magnetocaloric
particles (1-2-3-4).