4116
Feasibility of rapid diffusion-weighted imaging for monitoring of MR-guided prostate high intensity focused ultrasound ablation
Elena Kaye1 and Oguz Akin2
1Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
1Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Synopsis
MR-guided high-intensity focused ultrasound (HIFU) treatment of prostate cancer is a promising non-invasive approach. The outcomes of these treatments can be improved with more accurate visualization of ablative necrosis surrounding a tumor. Diffusion-weighted imaging (DWI) could provide valuable information about tissue viability without injection of contrast. However, currently DWI requires high number of excitations (NEX), averages, takes several minutes and is not practical for intraprocedural monitoring. To make DWI a practical tool for monitoring of prostate HIFU, we propose to replace high NEX DWI acquisitions, with NEX=2 acquisitions and subsequent deep-learning denoising of these image.
Introduction
Over the past decade, several studies reported on the potential value of monitoring diffusion changes to visualize the extent of ablative necrosis during high intensity focused ultrasound (HIFU) treatment of the prostate1-4. A study on HIFU ablation of canine prostate showed that apparent diffusion coefficient (ADC) irreversibly changed upon tissue undergoing ablative necrosis 1, 3. In a clinical study, immediate post-ablation diffusion weighted (DW) image displayed changes that spatially predicted ablation zone4. Despite its potential for thermal ablation monitoring, systematic investigation of diffusion weighted imaging (DWI) during HIFU is challenging because of the substantial increase in scan/procedure time that acquisition of DW images would require. Due to low signal-to-noise ratio (SNR) of prostate DW images, each acquisition takes several minutes as the large number of excitations (NEX) is performed to compensate for low SNR (for a b-value of 1000, NEX = 16). To make DWI a practical tool for monitoring of prostate HIFU, we propose to accelerate the conventional acquisition by leveraging image denoising in place of using large NEX and averaging.Methods
Our goal was to denoise prostate DW images obtained with NEX = 2 so that image quality of denoised images was comparable to images acquired using NEX = 16. We implemented and modified a denoising convolutional network (DnCNN)5, which was reported to have superior performance to the state-of-the-art image-prior-based methods. To train DnCNN, pairs of noisy and reference images are needed. Given that DW images are not routinely collected during prostate HIFU, an intraprocedural prostate DW data set was not available. Instead conventional diagnostic prostate DW images were used to train (N = 103 patients) and validate (N = 15) DnCNN. All slices were used. Figure1A shows data pre-processing steps. The input for the modified DnCNN was a pair consisting of a low-b-value image and a high-b-value 2-NEX image (Figure 1B). The output was a denoised image, computed as 2-NEX image minus the residual image. L2 loss between the denoised 2-NEX and reference 16-NEX DW images was used as the cost function. Validation set was used to set the optimal number of epochs, patch size and network’s depth. To investigate how well the denoising model would perform with images acquired during HIFU treatment, the resulting pre-trained model was applied to denoise previously not-seen by the model intraprocedural DW images containing an endorectal water balloon housing the HIFU transducer (1 patient, before-HIFU and after-HIFU sets, Figure 2). Figure 3 details imaging parameters. Denoising performance was evaluated on a separate 37-patient DWI test set: peak-SNR (PSNR) and the structural similarity index (SSIM) metrics were computed for 2-NEX and denoised 2-NEX DW images. Denoising of intraprocedural DW images was evaluated by investigating the difference between intraprocedural ADC and pre-treatment diagnostic scan’s ADC values. The values were measured in seminal vesicles, tumor and gluteal muscle (Figure 2). The paired-samples and independent-samples t-tests were used to compare the qualitative results (IBM SPSS Statistics 25). IRB approval was obtained for this retrospective study.Results
Based on the validation runs, the network depth was set to 20, patch size to 60x60, and number of epochs to 25. Batch size of 128 was used. The modified DnCNN, applied to the noisy 2-NEX prostate DW images, demonstrated excellent performance in a test set of 37 patients (Figure 4). Denoising significantly increased PSNR from 14.1 ± 2.6dB to 33.8 ± 3.6dB (p<0.01) and SSIM from 0.58 ± 0.07 to 0.93 ± 0.04 (p<0.01). The denoised 2-NEX DW images had comparable qualitative appearance to the reference 16-NEX DW images. Figure 5A shows the results of denoising of the intraprocedural DW images. Retrospective review of the MR-guided prostate HIFU image sets identified only one case, in which intraprocedural DWI was performed. The denoising model pre-trained on diagnostic prostate images generalized well to the new type of images, images containing endorectal balloon and HIFU transducer. No artifacts were created by denoising. Qualitatively, the conspicuity of seminal vesicles, bladder wall, tumor and gluteal muscle was increased by denoising on both before and after-HIFU DW images. Changes in DW and ADC images after thermal ablation are clearly visualized after denoising without having to acquire large NEX. The mean differences between diagnostic pre-treatment ADC values and intraprocedural ADC values in seminal vesicles, muscle and prostate tumor (Figure 5B) were reduced by 65%, 55% and 42% after applying denoising. Using NVIDIA Tesla K40 GPU, denoising time for a single slice was 0.006 s.Discussion
The results of this study demonstrate preliminary feasibility of using deep-learning denoising to accelerate acquisition of DW images during prostate HIFU. Reducing acquisition time via reducing NEX and applying denoising can make conventional DWI much more suitable for monitoring of HIFU and other prostate MR-guided therapies. Modern GPUs and carefully-trained deep-learning model enables sub-second computational times for denoising of an entire series, which is crucial for a treatment monitoring setting. While denoising does not address the geometric distortion of single-shot echo-planar DWI, it is a generalizable method, and in the future can be combined with multi-shot DWI which is less prone to distortion. Finally, large numbers of the diagnostic prostate DW images can be leveraged to train robust models for prostate therapy monitoring.Acknowledgements
We thank Dr John Pauly for valuable discussions, and James Keller for editorial assistance.References
1. Chen J, Daniel BL, Diederich CJ, et al. Monitoring prostate thermal therapy with diffusion‐weighted MRI. 2008;59(6):1365-72. 2. Partanen A, Yerram NK, Trivedi H, et al. Magnetic resonance imaging (MRI)‐guided transurethral ultrasound therapy of the prostate: a preclinical study with radiological and pathological correlation using customised MRI‐based moulds. 2013;112(4):508-16. 3. Plata JC, Holbrook AB, Marx M, et al. A feasibility study on monitoring the evolution of apparent diffusion coefficient decrease during thermal ablation. 2015;42(9):5130-7. 4. Ryan L Brunsing SH, Rachelle Bitton, Bruce Daniel, Geoffrey Sonn, Pejman Ghanouni, Post-treatment diffusion weighted imaging predicts ablation zone margins following MRI-guided high intensity focused ultrasound of the prostate. ISMRM; 2019; Montreal, Canada. 5. Zhang K, Zuo W, Chen Y, Meng D, Zhang L. Beyond a gaussian denoiser: Residual learning of deep cnn for image denoising. IEEE Transactions on Image Processing. 2017;26(7):3142-55.Figures

Figure 1. A. Data pre-processing schematic. For each
patient raw data was reconstructed to produce: 1) low-b-value images
reconstructed all available NEX (usually 2-4), 2) high-b-value images
reconstructed using 2 NEX (noisy) and all 16 NEX (reference image). B. Modified
DnCNN with all layers defined in Ref 5. Conv-convolution layer with rectified linear units (ReLU). Layers 2 to 19 have added batch normalization (BN).
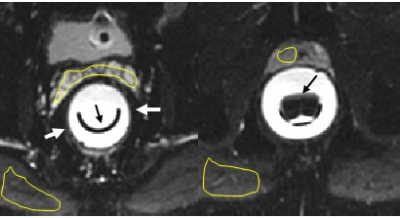
Figure 2. Representative
cropped slices from intraprocedural DWI (low b-value) showing endorectal balloon
(white arrows) housing HIFU transducer (black arrows). ADCs in various tissue
structures (seminal vesicles), gluteal muscle and prostate tumor, were measured using yellow hand-drawn contours.
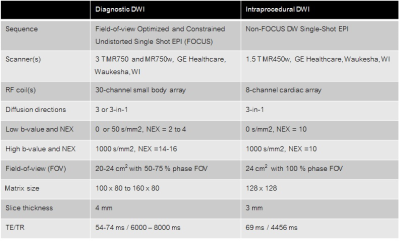
Table 1. Imaging parameters
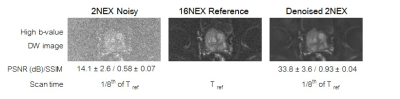
Figure 4. Representative
result of denoising showing DW images obtained with NEX=2 and NEX=16, and the
denoised 2NEX image. PSNR and SSIM (mean ± standard deviation) significantly improved by denoising. 2NEX DW
acquisition requires 1/8th of scan time needed for 16NEX DW scan.

Figure 5. A. Denoising of intraprocedural DW images obtained
during prostate HIFU. On DW images (left) denoising substantially improved visualization
of seminal vesicles and bladder wall (white arrows), muscle (*), tumor and ablation
zone (yellow arrows). ADC maps computed denoised images appear substantially
improved with enhanced visualization of tumor, and post-ablation tissue
changes. Window level is the same for the denoised and noisy images. B. Measured ADC values (mean ± standard deviation)