4115
Enhancement of the MRgHIFU therapy using endovascular sono-sensitizers demonstrated in an experimental model of perfused tissue1Image Guided Interventions Laboratory,University of Geneva, Faculty of Medicine, Geneva, Switzerland, 2University of Avignon, CBSA-IBMM (UMR5247), Avignon, France, 3Radiology Department, University Hospitals of Geneva,, Geneva, Switzerland, 4Research and Development Laboratory, Visceral and Transplantation Service, University Hospital Geneva, Geneva, Switzerland
Synopsis
Magnetic Resonance guided High Intensity Focused Ultrasound (MRgHIFU) is accepted for the non-invasive ablation of localized tumors. Thermal contrast between the target tissue and pre/post focal tissues can be improved for highly perfused tumors by injection of liquid core micro-droplets, used as endovascular sono-sensitizers. We are aiming to provide a substitution model for living organs or perfused ex vivo organs to facilitate the investigation of new sono-sensitizers and sonication paradigms. We are reporting an experimental model that is suitable for MR-guided HIFU studies, offering in situ tunable perfusion rate, multi-compartment structure, and tunable concentration of sono-sensitizers and dissolved gases.
Introduction
Magnetic Resonance guided High Intensity Focused Ultrasound (MRgHIFU) has been progressively accepted for thermal ablation of tumors. Ablation of highly perfused tumors is challenging due to the heat sink effect. Previous studies demonstrated an enhancement in the thermal effect at the focal point in tissue mimicking gel samples doped with liquid-core PFOB surrounded by biocompatible fluorinated surfactants, used to increase the absorption of the HIFU beam in the tissue [1]. Additional properties, e.g. dissolved oxygen, size of sono-sensitizers or HIFU parameters need to be optimized by exhaustive testing in ex vivo organs or living bodies. We developed a substitution model of 'artificial' kidney composed of perfused tissue-mimicking gel matrix.Material and method
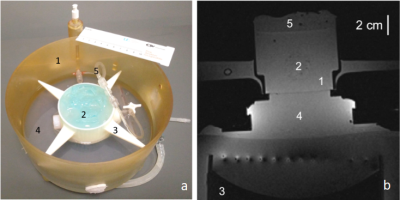
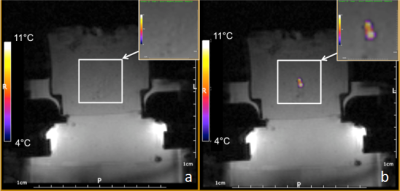
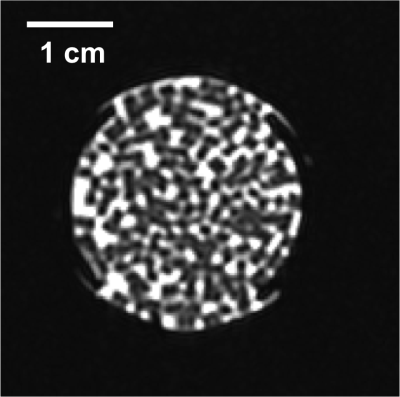
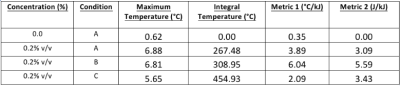
Tissue mimicking gel was prepared according to Menikou et al [2]. and sticks of 1 x 1 x 60 mm were chopped and densely packed (corresponding to 90% of tissue mimicking gel and 10% of circulating fluid) in a 1.5% v/v agar gel matrix, to mimic the micro-circulation in capillaries (Fig 1) . The main difficulty of such a preparation is the contamination with bubbles, which create a barrier for HIFU and affect the quality of MR images. To resolve, the gel matrix was prepared with degassed water, then gel sticks were packed under degassed water, and the 'artificial' kidney was further degassed 30 minutes by connecting to a vacuum circulating pump. The 'artificial' kidney was placed in a 3D-printed holder designed to avoid motion during HIFU shots and MR vibration from gradients. The device was perfused with an MR-compatible perfusion machine (Fig 1) delivering oxygen and perfusate [3]. Focused ultrasound was generated by an MR-compatible 256-element phased array transducer operating at 1MHz . Sono-sensitizers with 2.35µm average diameter (SD = 0.5µm) were prepared with 2% v/v of PFOB. One dose of 30mL (corresponding to 0.2%v/v of the total perfusion fluid volume) was injected in the perfusion fluid and two sonication protocols were conducted to demonstrate the efficiency of sono-sensitizers. The first protocol consisted of sonications at a fixed focal point with 94W of acoustic power during 20s and with 60W during 20s (respectively condition A and B). In the second protocol, the energy was delivered in a 2mm-diameter circle, described by 16 points, at 94W for a total duration of 33s (condition C). Experimental data were acquired in a whole-body Siemens 3T MRI scanner using a 11 cm diameter receive loop coil. Relative localization of the HIFU transducer and 'artificial kidney' was performed with a high-resolution T1-weighted 3D sequence (Fig 1). The temperature elevation was computed by the PRFS sensitive method (voxel size 1x1x4mm3) with a segmented GRE-EPI sequence in the axial plane through the focal point. MR images were reconstructed in real-time and magnitude images were merged with temperature maps see Fig 2. The microcirculation in the interstitial compartment between sticks and the gel impermeability to sono-sensitizers was assessed by injection of gadolinium chelate at the end of the experiment monitored with T1 weighted image (Fig 4). Two metrics were calculated, according to Desgranges et al. [4], to evaluate sono-sensitizers efficiency (Fig 5).Results
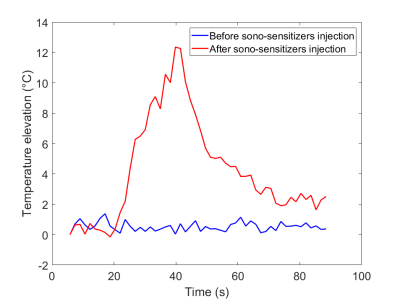
T1 weighted MR images before and after degassing showed a dense distribution of gel sticks and a significant reduction of air bubbles in the interstitial compartment. Injection of gadolinium chelate demonstrated an effective perfusion and the impermeability of both tissue mimicking and surrounding agar gel (Fig 4), indicating that the sono-sensitizers could not be trapped. Temperature maps clearly demonstrated an enhancement in the achieved temperature after injection of sono-sensitizers. Figure 5 shows the temperature elevation in a ROI of 2 pixels around the warmest point, after injection of sono-sensitizers. For the circular pattern, the temperature enhancement achieved was 5.65 °C. For the fixed focal point pattern, the temperature enhancement achieved was 6.81 °C (Fig 3). The achieved temperature difference and the metrics before and after injection clearly demonstrated the sono-sensitizers efficiency. Metric#1 was calculated to be 2.09 °C/kJ and metric#2 was 3.43 J/kJ with 0.02% of sono-sensitizers in the total volume of capillaries (0.2% added in 10% circulating liquid ratio).Discussion
The fluid circulation in the artificial kidney allowed a renewal of fresh sono-sensitizers at the focal spot, enhancing the delivery method. Experimental data demonstrated the feasibility of the method by injection of sono-sensitizers in perfused gels. Unlike living organs, the interstitial volume of perfusate is pre-determined. Using the same HIFU parameters, Desgranges et al. reported very similar values for metric#2 using a volume fraction of 0.1% v/v of sono-sensitizers versus total gel volume, whereas our volume fraction of 0.2% is provided versus the perfusate volume meaning 0.02% v/v of the 'organ' volume, that is, an approximate factor of 5 stronger enhancement.Conclusion
The model demonstrated effective perfusion confined in the interstitial compartment, and enhancement in the temperature elevation induced by HIFU after injection of a fraction of sono-sensitizers in the perfusion fluid. Adding 0.02% sono-sensitizers in total volume of a perfused 'artificial kidney' demonstrated a 5.65°C enhancement in the heating for the circular pattern and 6.81°C for the fixed focal point pattern. This 'artificial kidney' is a relevant model of a perfused organ that can be used in the future for optimization of HIFU energetics and treatment conditions.Acknowledgements
No acknowledgement found.References
[1] Astafyeva K, Somaglino , Desgranges D, Berti R, Patinote C, Langevin D, Lazeyras F, Salomir R, Polidori A, Contino-Pépin C, Urbachij W, Taulier N. Perfluorocarbon nanodroplets stabilized by fluorinated surfactants: characterization and potentiality as theranostic agents. J Mater Chem B 2015; 3:2892-2907.
[2] Menikou G, Damianou C. Acoustic and thermal characterization of agar based phantoms used for evaluating focused ultrasound exposures. J Ther Ultrasound. 2017; 5: 14.
[3] Buchs JB, Bühler L, Morel P. A new disposable perfusion machine, nuclear magnetic resonance compatible, to test the marginal organs and the kidneys from non-heart-beating donors before transplantation. Interactive CardioVascular and Thoracic Surgery 6 (2007) 421–424
[4] Desgranges S, Lorton O, Gui-Levy L, Guillemin P, Celicanin Z, Hyacinthe JN, Breguet R, Crowe LA, Becker CD, Soulié M, Taulier N, Contino-Pépin C, Salomir R. Micron-sized PFOB liquid core droplets stabilized with tailored-made perfluorinated surfactants as a new class of endovascular sono-sensitizers for focused ultrasound thermotherapy. J. Mater. Chem. B, 2019,7, 927-939
Figures