4114
MR imaging Evaluation of an Interstitial Focused Ultrasound Probe used in Robotically Assisted Brain Ablation Procedures1GE Global Research, Niskayuna, NY, United States, 2Acoustic MedSystems Inc., Savoy, IL, United States, 3WPI, Worcester, MA, United States, 4Neurosurgery, Albany Medical College, Albany, NY, United States
Synopsis
In this work we evaluate a newly developed catheter-based therapeutic ultrasound (US) probe used for interstitial MR-guided high intensity focused US (iMRgFUS) procedures. Specifically, we optimize and down-select MR thermal imaging protocols in order to achieve maximum temperature measurement precision and volume coverage in the presence of image artifacts arising from the iMRgFUS probe design and operation.
Purpose
Thermotherapy-based interventional technologies have rapidly developed as means to treat several pathologies. Ablative therapies have become especially useful in the treatment of movement disorders and brain tumors.[1-3] These procedures are typically monitored by magnetic resonance temperature imaging (MRTI), using the proton resonance frequency (PRFS) method.[4] The PRFS method is very sensitive to image phase artifacts, and therefore much care must be given to ablation probe design to minimize susceptibility-induced image artifacts. Interstitial MR-guided focused ultrasound (iMRgFUS) probes contain metal wires, piezoelectric material and water flow lines for cooling, all of which have the potential to cause drastic MRTI degradation. Here we (i) perform quantitative ΔB0, SNR, B1+ phantom tests on a recently developed catheter based iMRgFUS probe [5] in various orientations and power states, and (ii) perform phantom MRTI heating validation tests at multiple sonication power levels using 4 different MRTI sequences. The goal of this work is to evaluate, optimize and down-select MRTI protocols in order to achieve maximum temperature measurement precision and volume coverage in the presence of image artifacts arising from the iMRgFUS probe design and operation.Methods
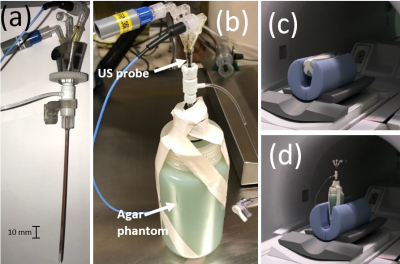
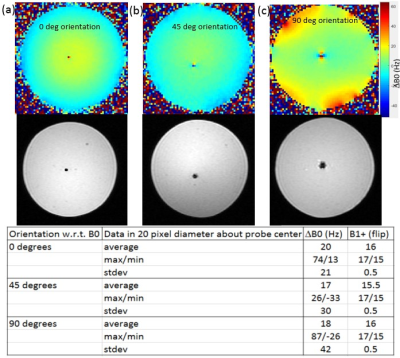
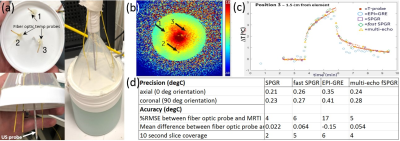
Phantom imaging tests were performed on a 3T GE Architect scanner (GE Healthcare, Waukesha, WI). Body coil transmit was used with a single channel (15 cm ID) loop coil for receive signal. Phantoms were 3% Agar solution doped with copper sulfate to mimic the relaxivity of brain tissue at 3T. Two separate phantoms were used for testing: one phantom containing only the probe in Agar as depicted in Fig. 1 (phantom 1), and one containing multiple fiber optic temperature probes for MRTI validation testing seen in Fig. 3a (phantom 2). The iMRgFUS probe (Acoustic Medsystems, Savoy, IL) was constructed with a multielement 10mm long tubular piezoelectric transducer mounted on a hollow polyimide tubular structure. It was inserted into the Agar phantom as can be seen in Fig. 1a. Quantitative imaging tests were performed to assess (i) ΔB0, (ii) B1+, and (iii) basic SNR tests using a gradient-echo (GRE) sequence. All imaging tests were performed at 0°, 45°, and 90° orientations of the axis of the catheter based therapeutic US probe with respect to the main magnetic field (B0). Figs. 1c-d depicts the parallel (0°) and orthogonal (90°) orientation examples. Phantom MRTI tests were performed using phantom 2 depicted in Fig. 3a with three fiber optic temperature probes (Neoptix) distributed in-plane, at different radial distances from the US transducer element. Four sequences were tested using the PRFS MRTI method: a spoiled gradient echo (SPGR), echo-planar imaging -GRE, fast SPGR, and multi-echo fast-SPGR. The precision and accuracy of MRTI measurements are defined as the standard deviation of the temperature measurements averaged within a 20-pixel diameter region centered on the probe, and the absolute difference between the temperature probe readings and the MRTI reading at the T-probe locations respectively.Results and discussion
Phantom 1 was constructed with only the US probe and Agar in order to minimize any potential confounding artifacts during ΔB0, SNR, B1+ measurements. These imaging measurements were repeated with phantom 2, and no new imaging artifacts were noted when the temperature probe was inserted. The table in Fig. 2 summarizes the results of the orientation dependence study for phantom 1. The standard deviation of ΔB0 is reduced by a factor of 2 in the parallel (0°) orientation. SNR likewise increases within the 20-pixel diameter averaging region in the parallel position, indicating that ΔB0 effects primarily contribute to MR signal drop in the immediate vicinity of the US probe. B1+ maps remain largely unchanged in the different orientations. The table in Fig. 3 indicates the precision, accuracy, and maximum volume coverage in a 10 second window for the multiple MRTI methods tested in phantom 2. Overall, single-echo SPGR provided the highest temperature precision and accuracy, although it performs worst in terms of volume coverage. Relative to SPGR, EPI-GRE provides a factor of 3 increase for volumetric coverage yet underperforms in terms of MRTI temperature precision and accuracy (over 40% reduction). Fast-SPGR, and multi-echo fast-SPGR perform similarly well, and give similar MRTI metrics with an approximate 2-fold increase in volumetric coverage relative to SPGR.Acknowledgements
This work was funded in part by NIH 2R01CA166379References
1. Kangasniemi M, Diederich CJ, Price RE, et al. Multiplanar MR temperaturesensitive imaging of cerebral thermal treatment using interstitial ultrasound applicators in a canine model. J Magn Reson Imaging. 2002;16(5):522-531.
2. Herickhoff CD, Light ED, Bing KF, et al. Dual-mode intracranial catheter integrating 3D ultrasound imaging and hyperthermia for neuro-oncology: feasibility study. ltrason Imaging. 2009;31(2):81-100.
3. Canney MS, Chavrier F, Tsysar S, Chapelon JY, Lafon C, Carpentier A. A multi-element interstitial ultrasound applicator for the thermal therapy of brain tumors. J Acoust Soc Am. 2013;134(2):1647-1655.
4. Ishihara Y, Calderon A, Watanabe H, Okamoto K, Suzuki Y, Kuroda K, Suzuki Y. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med 1995;34:814–823.
5. J. MacDonell, N. Patel, G. Fischer, E. C. Burdette, J. Qian, V. Chumbalkar, G. Ghoshal, T. Heffter, E. Williams, M. Gounis, R. King, J. Thibodeau, G. Bogdanov, O. W Brooks, E. Langan, R. Hwang, J. G Pilitsis, “Robotic Assisted MRI-Guided Interventional Interstitial MR-Guided Focused Ultrasound Ablation in a Swine Model,” Neurosurgery, 2019;84(5), 1138–1148.
Figures