4113
Intraoperative Diffusion Weighted MR Image Contrast during Focused Ultrasound Ablation in a Preclinical Swine Model1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Neurosurgery, University of Virginia, Charlottesville, VA, United States, 3Center for Comparative Medicine, University of Virginia, Charlottesville, VA, United States, 4Radiology, Stanford University, Stanford, CA, United States, 5Radiology, University of Virginia, Charlottesville, VA, United States
Synopsis
This abstract presents a preclinical study of intraoperative diffusion weighted imaging (DWI) of focused ultrasound thermal lesions in the thalamus using a novel acquisition sequence. We found that DW imaging can visualize thermal lesions within 10 minutes of ablation. Our study indicates that MR-guided focused ultrasound lesions present a rapid and persistent decrease in apparent diffusion coefficient within minutes after ablation and that diffusion-based lesion classification methods can outperform T2-w based methods.
Introduction
Transcranial MR-guided focused ultrasound (T-MRgFUS) is an image-guided, non-invasive surgery for essential tremor that uses a steerable transducer array to deposit 10-15 s duration bursts of acoustic energy into a precise volume of the patient’s thalamus1. Currently, ET symptom reductions after T-MRgFUS do not reach the same levels of efficacy as deep brain stimulation2. There is a critical need to improve T-MRgFUS treatment efficacy by ensuring that permanent tissue necrosis occurs within the entire ablation target.Currently, ET lesions develop T2-weighted (T2-w) contrast very slowly3, prohibiting intraoperative lesion assessment. Meanwhile, diffusion-weighted imaging (DWI) has previously been proposed to provide intraoperative detection of thermal ablation in the brain4. There is significant indirect evidence supporting this hypothesis, including DWI as early as 30 minutes post ablation5. While previous reports are promising, they do not test whether DWI contrast can be observed prior to the development of any T2 lesion contrast or whether it can better intraoperatively predict lesion formation. Here, we present a novel pulse sequence that can acquire intraoperative DW images in a T-MRgFUS porcine model. We then compare the resulting DW images to intraoperative T2-w images.
Methods
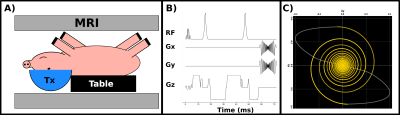
Experiments were conducted using a porcine craniotomy model6, which is displayed in Figure 1a. The heads of six animals were positioned in a supine orientation at the focus of a 1024 element, 650 kHz, electronically steered transducer system (ExAblate Neuro, INSIGHTEC, Haifa, Israel) and scanned inside a 3T MR scanner (GE MR750, General Electric, Waukesha, WI) using a custom-built transmit-receive surface coil.Two to three coplanar targets in each hemisphere of the thalamus were located by the ultrasound device, for an average of 4.33 targets per animal and a total of 26 targets. Each target was sequentially selected and confirmed by depositing 20-40 W of acoustic power for 10 s, generating sub-ablative temperature increases. After confirmation, the transmit power, and, if necessary, the sonication durations were increased over 2-7 sonications, depending on the subject and the target, until the peak estimated temperature, as measured by simultaneous MR thermometry using a clinically established sequence7, reached or exceeded 60 oC. After ablation, the lesion was continuously monitored for 35 minutes using a custom DW imaging sequence. Prior to treatment, a control monitoring session was performed as a means to compute baseline means and standard deviations of T2-w and DW image contrasts as well as of the ADC of each target.
DWI monitoring of each lesion was completed using a multi-slice, self-navigated spiral sequence8 with several adaptations to the intraoperative environment. The spiral sequence employed a variable density, retraced readout to reduce image blur8,9 and a twice refocused, adiabatic RF pulsing scheme to reduce both eddy currents and B1 inhomogeneity10. Fat suppression was accomplished using a spectral-spatial RF excitation pulse. A schematic of the sequence is shown in Figure 1.B. All images were reconstructed using a single-channel coil adaptation of a multi-shot DW iterative reconstruction method10. Sequence parameters are: TE/TR: 60/1500 ms; Slice Thickness/Gap = 5/1 mm; Matrix Size: 200 x 200 x 3; FOV: 300 mm; Spiral Interleaves/Readout Duration: 44/11 ms; bvalue: 815 s mm-2.
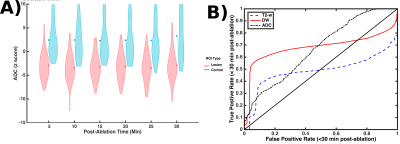
Regions of interest (ROI) in the resulting images were selected about each visible lesion by averaging over a 3x3 pixel grid placed about the center-most pixel of each lesion as it appeared on DWI scans taken 30 minutes after ablation. Untreated locations in the cortex were also selected as controls. T2-w, DW contrast levels and ADC estimates were computed for each ROI and subtracted from their respective means calculated from the baseline images. To normalize the resulting data, these differences were transformed to z-scores by scaling each difference by its ROI's respective standard deviation. Because imaging sessions were interleaved between sequential treatments, many z-scores were derived from targets that had not yet been ablated. Receiver operator characteristic (ROC) curves were calculated by using a series of z-score values as lesion detection thresholds for the ADC, DW, and T2-w ROI’s and then computing the resulting false positive and true positive rates.
Results
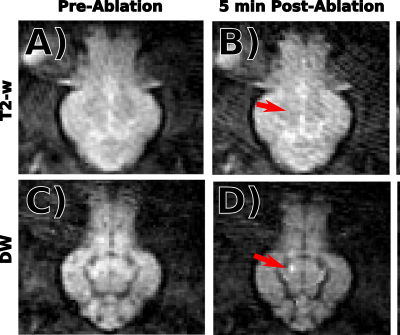
In 20 out of 26 cases, DW contrast appeared appear before or simultaneously with T2-w contrast. In the remaining six cases, image quality was too poor or the lesions were too small to be identified in either T2-w or DW images. Example T2-w and DWI images taken after two separate ablations in the same subject are displayed in Figure 2. DW contrast clearly precedes T2-w contrast. Aggregated ADC z-scores are displayed as violin plots in Figure 3a, which show an early and sustained decrease in ADC for most targets after ablation. The ROC curves displayed in Figure 3b show that DWI and ADC based lesion classification outperform T2-w based classification for most cases.Discussion and Conclusion
Our results indicate that T-MRgFUS lesions in thalamic tissue present a rapid and persistent decrease in ADC within minutes after ablation and that intraoperative diffusion-based lesion classification methods can outperform T2-w based methods. Future work should address pertinent clinical questions such as whether DWI and ADC lesion contrasts remain stable over the first 24 hours after ablation and whether a tissue diffusion model can predict tremor reduction or adverse events.Acknowledgements
This work is supported by the Focused Ultrasound Foundation.References
1. Elias, W. Jeffrey, Nir Lipsman, William G. Ondo, Pejman Ghanouni, Young G. Kim, Wonhee Lee, Michael Schwartz, et al. 2016. “A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor.” New England Journal of Medicine375 (8): 730–39
2. 8. Boutet A, Ranjan M, Zhong J, et al. Focused ultrasound thalamotomy location determines clinical benefits in patients with essential tremor. Brain 2018;141:3405–3414.
3. Chen L, Bouley D, Yuh E, D’Arceuil H, Butts K. Study of focused ultrasound tissue damage using MRI and histology. J. Magn. Reson. imaging 1999;10:146–153.
4. Mórocz IÁ, Hynynen K, Gudbjartsson H, Peled S, Colucci V, Jólesz FA. Brain edema development after MRI-guided focused ultrasound treatment. J. Magn. Reson. Imaging 1998;8:136–142.
5. Chazen JL, Stradford T, Kaplitt MG. Cranial MR-guided Focused Ultrasound for Essential Tremor. Clin. Neuroradiol. 2019;29:351–357.
6. Elias WJ, Khaled M, Hilliard JD, Aubry JF, Frysinger RC, Sheehan JP, Wintermark M, Lopes MB. A magnetic resonance imaging, histological, and dose modeling comparison of focused ultrasound, radiofrequency, and Gamma Knife radiosurgery lesions in swine thalamus. J Neurosurg 2013;119:307–317.
7. Lipsman, Nir, Michael L. Schwartz, Yuexi Huang, Liesly Lee, Tejas Sankar, Martin Chapman, Kullervo Hynynen, and Andres M. Lozano. 2013. “MR-Guided Focused Ultrasound Thalamotomy for Essential Tremor: A Proof-of-Concept Study.” The Lancet. Neurology12 (5): 462–68.
8. Fielden, Samuel W., and Craig H. Meyer. 2015. “A Simple Acquisition Strategy to Avoid Off-Resonance Blurring in Spiral Imaging with Redundant Spiral-in/out k-Space Trajectories.” Magnetic Resonance in Medicine73 (2): 704–10
9. Allen, Steven P., Xue Feng, Samuel W Fielden, and Craig H Meyer. 2019. “Correcting Image Blur in Spiral, Retraced in/out (RIO) Acquisitions Using a Maximized Energy Objective.” Magnetic Resonance in Medicine81 (3): 1806–17.
10. Auerbach E, Ugurbil K. Improvement in Diffusion MRI at 3T and Beyond with the Twice-Refocused Adiabatic Spin Echo (TRASE) Sequence. In: Proceedings of the 12th Annual Meeting of ISMRM. ; 2004. p. 2464.
Figures