4111
A method for post-surgical evaluation of targeting accuracy in transcranial focused ultrasound thalamic ablation1Department of Radiology & Medical Imaging, Division of Neuroradiology, University of Virginia Health System, University of Virginia, Charlottesville, VA, United States, 2Brain Institute, University of Virginia, Charlottesville, VA, United States
Synopsis
MR-guided focused ultrasound ablation targets small thalamic nuclei that are frequently difficult to distinguish from surrounding tissue. The ability to accurately evaluate the success in ablating the target nuclei is essential for predicting treatment outcome and evaluating surgical performance. We show microstructural changes in the thalamus that may prevent accurate post-surgical tractography. We propose a straightforward technique that reconstructs neuronal connections in the pre-surgical brain using the post-operative lesioned area as a seed-region for constrained spherical deconvolution based tractography. The proportion of tracts leading to regions known to be connected to the target nuclei is then evaluated.
Introduction
Transcranial focused ultrasound (FUS) ablation in the thalamus received FDA approval for the treatment of medication refractory essential tremor in 2016 and is currently being explored as a treatment option in a number of other neurological disorders1,2. Accurate targeting of the high-frequency FUS ablation is important for successful treatment outcomes as well as avoiding adverse effects such as blood vessel injury3. Studies have also reported a range of responses to treatment up to relapse from treatment and return of presurgical symptoms, particularly in Parkinson’s disease patients4,5. A quantitative method to evaluate surgical accuracy could aid in predicting treatment outcome and potentially allow for early intervention. Prior methods of assessing FUS accuracy either relied on stereotaxic coordinate tracking3, or diffusion-tensor based tract reconstruction of connections to the target nuclei6,7. We propose utilizing constrained spherical deconvolution as a superior basis for tractography and the use of the baseline scans to avoid transient post-operative microstructural changes.Methods
Retrospective diffusion MRI data was taken from 12 patients who participated in a previously published open-label pilot study of FUS thalamotomy for the treatment of medication-refractory essential tremor at the University of Virginia1. Patients underwent MRI scans prior to the procedure and at 1 day, 1 week, 1 month, and 3 months post-operatively. T1-weighted images and diffusion weighted images were collected at each timepoint, with the T1-weighted images having a voxel size of 0.9x0.45x0.45mm3 and dimensions 240x512x512 and the diffusion-weighted images having a voxel size of 1.8x1.8x5.2mm3 and dimensions 128x128x30 with 4 directions at b=0 and 80 directions at b=1000s/mm2. Diffusion images were analyzed using the constrained spherical deconvolution-based, single-shell, 3 tissue algorithm (SS3T-CSD)8 implemented in the open source software MRtrix9. Several preprocessing steps utilized FSL10. Diffusion images were denoised11, corrected for Gibbs ringing12, susceptibility distortions13, motion14, and eddy currents15. Average response functions were generated for white matter (WM), grey matter (GM), and CSF from the images in the study16 and the fiber orientation distribution (FOD) calculated for each voxel8. 3-tissue signal fractions were calculated from the FODs17. To delineate voxels that underwent FUS ablation, we manually masked lesions on T1-weighted images collected at 1 day and 1 week post-surgery. At these time points the lesion is characterized as a dark region with clear contrast to surrounding tissue. T1-weighted whole brain images were then registered with pre-surgical baseline images using the SyN algorithm from ANTs18 allowing for masks to be transformed into each subject’s baseline native space. Lesion masks were then used as the seed region for tractography with SS3T-CSD generated WM FODs from the baseline diffusion image. Tractography was performed using the iFOD2 algorithm19 to generate 50,000 bi-directional individual tracts which were subsequently filtered based on the FOD using the SIFT algorithm20 to arrive at a final result of 10,000 tracts. The target of FUS ablation in this cohort was the left ventral intermediate (VIM) nucleus of the thalamus8 which is part of a neuronal circuit with the motor cortex21. An independently developed motor cortex ROI22 was used to assess how many tracts, as a fraction of the final 10,000, were accurately able to model the WM connections between the VIM and motor cortex.Results
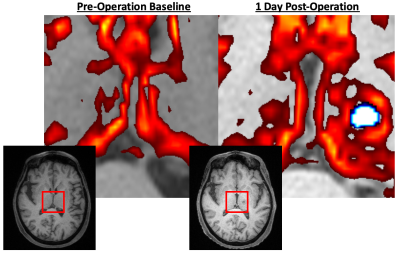
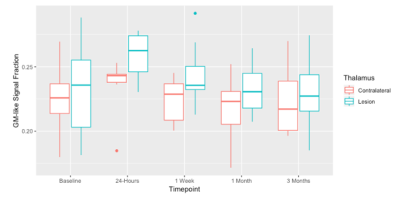
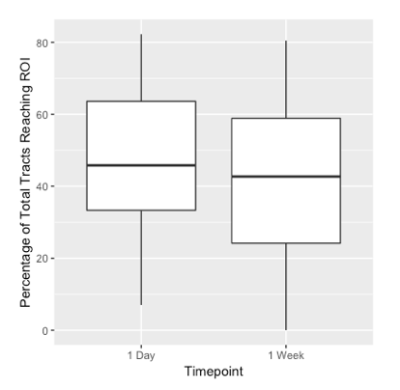
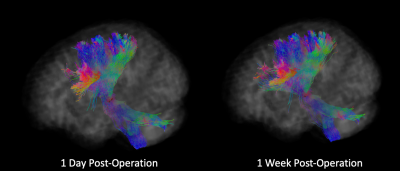
There was a significant increase in the GM-like signal fraction in the lesioned thalamus compared to the same individuals’ contralateral thalamus post-operation from baseline (ANOVA, F1,100 = 8.35, p<0.01). This increase occurred proximal to the lesion observed in T1-weighted images and extended out over a wider portion of the thalamus (Figure 1). The GM-like signal fraction results from patterns of isotropic diffusion, and thus represents a change in cellular microstructure that would impact the ability of tractography (being reliant on anisotropic diffusion patterns) to reconstruct axons in the affected area. This increase appears transient, with the average GM-like signal fraction decreasing from 1 day post-operatively to 3 months (Figure 2).Tractography results showed that an average of 44.7% (±7.2 SE) of tracts reached the target ROI from the lesioned area defined at 1-day post-operation and an average of 41.4% (±7.8 SE) reached the target ROI from the lesioned area defined at 1-week. There was no significant difference (ANOVA, F1,20 = 0.373, p=0.55 n.s.) between the number of tracts that reached the target ROI from the lesioned area at these two time points (Figure 3). Tracts extended bi-directionally from the target area, and though it was not quantitatively investigated it can be observed that the method described in this study is able to reconstruct both ascending and descending projections from the VIM nucleus to the motor cortex and cerebellum/brainstem, respectively (Figure 4).
Conclusion
This study has demonstrated a method for quantitatively measuring the targeting accuracy of FUS thalamotomy for the treatment of essential tremor. By using advanced diffusion imaging analysis techniques, detailed tractography and quantitative evaluation of cellular microstructure was able to be performed in clinical quality MRI data collected in 2011. This study also finds evidence for post-operation changes in brain microstructure in the thalamus that should be further investigated in the context of post-operation evaluation.Acknowledgements
No acknowledgement found.References
1. Elias, W. J., Huss, D., Voss, T., Loomba, J., Khaled, M., Zadicario, E., ... & Druzgal, J. (2013). A pilot study of focused ultrasound thalamotomy for essential tremor. New England Journal of Medicine, 369(7), 640-648.
2. Krishna, V., Sammartino, F., & Rezai, A. (2018). A review of the current therapies, challenges, and future directions of transcranial focused ultrasound technology: advances in diagnosis and treatment. JAMA neurology, 75(2), 246-254.
3. Jeanmonod, D., Werner, B., Morel, A., Michels, L., Zadicario, E., Schiff, G., & Martin, E. (2012). Transcranial magnetic resonance imaging–guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurgical focus, 32(1), E1.
4. Schlesinger, I., Eran, A., Sinai, A., Erikh, I., Nassar, M., Goldsher, D., & Zaaroor, M. (2015). MRI guided focused ultrasound thalamotomy for moderate-to-severe tremor in Parkinson’s disease. Parkinson’s Disease, 2015.
5. Schlesinger, I., Sinai, A., & Zaaroor, M. (2017). MRI-guided focused ultrasound in Parkinson’s disease: a review. Parkinson’s Disease, 2017.
6. Wintermark, M., Huss, D. S., Shah, B. B., Tustison, N., Druzgal, T. J., Kassell, N., & Elias, W. J. (2014). Thalamic connectivity in patients with essential tremor treated with MR imaging–guided focused ultrasound: in vivo Fiber tracking by using diffusion-tensor MR imaging. Radiology, 272(1), 202-209.
7. Chazen, J. L., Sarva, H., Stieg, P. E., Min, R. J., Ballon, D. J., Pryor, K. O., ... & Kaplitt, M. G. (2017). Clinical improvement associated with targeted interruption of the cerebellothalamic tract following MR-guided focused ultrasound for essential tremor. Journal of neurosurgery, 129(2), 315-323.
8. Dhollander, T., & Connelly, A. (2016). A novel iterative approach to reap the benefits of multi-tissue CSD from just single-shell (+ b= 0) diffusion MRI data. In Proc ISMRM (Vol. 24, p. 3010).
9. Tournier, J. D., Smith, R., Raffelt, D., Tabbara, R., Dhollander, T., Pietsch, M., ... & Connelly, A. (2019). MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage, 116137.
10. Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W., & Smith, S. M. (2012). FSL. NeuroImage, 62(2), 782-790.
11. Veraart, J., Fieremans, E., & Novikov, D. S. (2016). Diffusion MRI noise mapping using random matrix theory. Magnetic resonance in medicine, 76(5),1582-1593.
12. Kellner, E., Dhital, B., Kiselev, V. G., & Reisert, M. (2016). Gibbs‐ringing artifact removal based on local subvoxel‐shifts. Magnetic resonance in medicine, 76(5), 1574-1581.
13. Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E., Johansen-Berg, H., ... & Niazy, R. K. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, S208-S219.
14. Andersson, J. L., Graham, M. S., Zsoldos, E., & Sotiropoulos, S. N. (2016). Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. NeuroImage, 141, 556-572.
15. Andersson, J. L., & Sotiropoulos, S. N. (2016). An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage, 125, 1063-1078
16. Dhollander, T., Raffelt, D., & Connelly, A. (2016). Unsupervised 3-tissue response function estimation from single-shell or multi-shell diffusion MR data without a co-registered T1 image. In ISMRM Workshop on Breaking the Barriers of Diffusion MRI (Vol. 5).
17. Newman, B. T., Dhollander, T., Reynier, K. A., Panzer, M. B., & Druzgal, T. J. (2019). Test-retest reliability and long-term stability of 3-tissue constrained spherical deconvolution methods for analyzing diffusion MRI data. bioRxiv, 764506.
18. Avants, B. B., Tustison, N., & Song, G. (2009). Advanced normalization tools (ANTS). Insight j, 2, 1-35.
19. Tournier, J. D.; Calamante, F. & Connelly, A. (2010). Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. Proceedings of the International Society for Magnetic Resonance in Medicine, 2010, 1670
20. Smith, R. E.; Tournier, J. D.; Calamante, F. & Connelly, A. (2013). SIFT: Spherical-deconvolution informed filtering of tractograms. NeuroImage, 67, 298-312
21. Fang, W., Chen, H., Wang, H., Zhang, H., Puneet, M., Liu, M., ... & Lu, X. (2016). Essential tremor is associated with disruption of functional connectivity in the ventral intermediate nucleus—motor cortex—cerebellum circuit. Human brain mapping, 37(1), 165-178.
22. Archer, D. B., Vaillancourt, D. E., & Coombes, S. A. (2017). A template and probabilistic atlas of the human sensorimotor tracts using diffusion MRI. Cerebral Cortex, 28(5), 1685-1699.
Figures