4110
A 13C/31P surface coil to visualize metabolism and energetics in the brain1Department of Radiology, University of California San Francisco, San Francisco, CA, United States, 2Center for Advanced Imaging Innovation and Research, and Center for Biomedical Imaging, New York University School of Medicine, New York, NY, United States, 3Department of Hematology-Oncology, Beth Israel Deaconess Medical Center, Boston, MA, United States, 4Department of Radiology, Beth Israel Deaconess Medical Center, Boston, MA, United States
Synopsis
In this work, we constructed a 13C/31P surface coil for studying cancer metabolism and bioenergetics in the brain. In a single scan session, hyperpolarized 13C-pyruvate MRS and 31P MRS were carried out for a healthy rat brain. A commercial volume proton coil was used for anatomical localization and B0 shimming. Results show good detection of 13C labeled lactate, alanine, and bicarbonate in addition to ATP, phosphocreatine, inorganic phosphate, phosphodiesters and phosphomonoesters from 31P MRS. The coil enables obtaining complementary information within a scan session, thus reducing the number of trials and minimizing biological variability for studies of metabolism and bioenergetics.
PURPOSE:
Hyperpolarized Carbon (13C) MR spectroscopy allows for in vivo measurements of metabolic conversion including 13C-pyruvate to lactate, which is upregulated in brain cancer1. Phosphorus (31P) MRS can be used to quantify ATP levels, allowing for complementary monitoring of bioenergetics in tumors2. In this work, we constructed a dual tuned 13C/31P coil for acquiring metabolism and energetics data to study cancer metabolism in a single setting. As a first step towards this goal, we tested the coil on a healthy rat brain.METHODS:
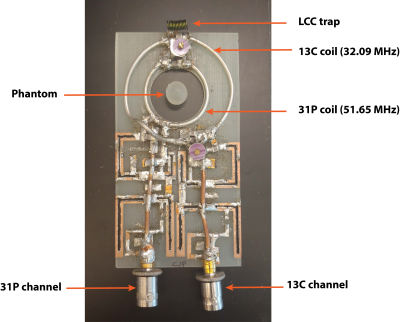
Experiments were carried out on a 3 T animal MRI scanner (Biospec, Bruker, Billerica MA), and animal experiments were done in accordance to the IACUC. The 13C/31P coil was constructed such that the inner loop was tuned to 51.65 MHz (31P frequency) and the outer loop was tuned to 32.09 MHz (13C frequency) and matched to 50 Ohms when loaded with a saline phantom (Fig 1). An LCC trap circuit3, consisting of an inductor and capacitor in parallel with another capacitor, on the 13C coil was used to block currents at the 31P frequency to decouple the two channels of the multinuclear coil. Tuning and matching were adjusted to obtain input power reflection less than 20 % at the respective center frequency inside the scanner. Either the 13C or 31P channel was connected to the X-nucleus channel on the scanner, while the other channel was terminated with a 50 Ohm load. Anatomical localization and B0 shimming were carried out using a volume proton (1H) coil (72 mm diameter, Bruker, MA). A coil file allowing for changing between the 1H, 13C, and 31P channels was constructed in ParaVision 6.0.1.For adjusting center frequency and system reference power for both channels, a spherical phantom (~200uL: 2M 1-13C-sodium acetate, 2M diethyl(2-oxopropyl) phosphonate) was positioned at the center of the coil (Fig 1). Diethyl(2-oxopropyl) phosphanate was specifically chosen so that its phosphorus spectrum does not overlap with in vivo spectra. The system reference power for a 90 degree flip angle was calculated by varying the transmit power of a series of single pulse acquisitions. In order to visualize the conversion of pyruvate to its downstream metabolic products in vivo, a bolus of hyperpolarized [1-13C] pyruvate (Testbed, Oxford Instruments, Oxfordshire UK) was administered to a healthy rat via tail vein. Slice selective spectroscopic sequences were used to obtain 13C (flip angle = 30 degrees, slice thickness = 10 mm, TR = 3s, repetitions = 64, spectral points = 2048, spectral bandwidth = 6421.23 Hz) and 31P (flip angle = 60 degrees, slice thickness = 15 mm, TR = 4s, averages = 256, spectral points = 2048, spectral bandwidth = 6421.23 Hz) spectra through the brain during a single scan session. Data were reconstructed in Mnova (MestReNova, 14.1.0).
RESULTS:
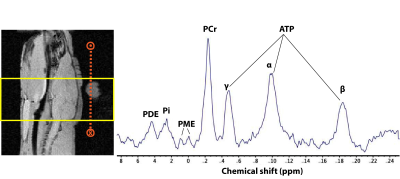
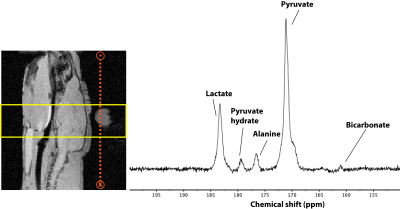
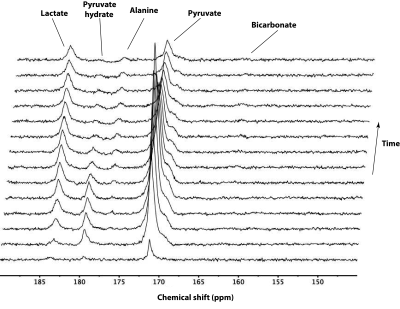
The Phosphorus spectrum in the brain showed clear Phosphocreatine and ATP peaks. In addition, inorganic phosphate, phosphomonoesters, and phosphodiesters were also observed (Fig 2). The summed (Fig 3) and first 14 time frames (Fig 4) of the carbon spectra show the downstream metabolic products of 13C-pyruvate, namely lactate, alanine, and bicarbonate. Slice positioning for both phosphorus and carbon spectroscopic acquisitions are shown in the sagittal anatomical slice obtained with the proton volume coil (Fig 2 and 3).DISCUSSION:
A number of dual tuned coil arrays have been constructed to allow for proton imaging for anatomical localization as well as X-nucleus imaging4,5. This work demonstrates the implementation of a 13C/31P coil in combination with an existing volume 1H coil to visualize metabolism, energetics, and anatomy. A nested loop design for the 13C/31P coil was chosen over a single loop design with two resonances in order to include an LCC trap on the lower frequency coil, which has been shown to improve coil sensitivity3. Future work on the coil includes the addition of cable traps for both channels to minimize common mode currents on the cables, and implementation of a switch to change between the 31P and 13C channels.CONCLUSIONS:
Non-invasive visualization of 13C labeled pyruvate and its downstream products in conjunction with 31P spectroscopy in the rat brain can be achieved using a 13C/31P coil.Acknowledgements
This work was supported by NIH Training Grant T32CA151022, American Cancer Society Research Scholar Grant 18-005-01-CCE, NIH grant P41 EB013598 and P41 EB017183. The authors would like to thank Rohan Virgincar for discussions regarding creating multinuclear coil files in ParaVision.References
1. Miloushev VZ, Granlund KL, Boltyanskiy R, Lyashchenko SK, DeAngelis LM, Mellinghoff IK, Brennan CW, Tabar V, Yang TJ, Holodny AI, Sosa RE, Guo YW, Chen AP, Tropp J, Robb F, Keshari KR. Metabolic Imaging of the Human Brain with Hyperpolarized 13C Pyruvate Demonstrates 13C Lactate Production in Brain Tumor Patients. Cancer Research 2018;78(14):3755-3760.
2. de Graff, R. A., In Vivo NMR Spectroscopy – Static Aspects. In Vivo NMR Spectroscopy. 2007; p 43-110.
3. Meyerspeer M, Roig ES, Gruetter R, Magill AW. An improved trap design for decoupling multinuclear RF coils. Magnetic Resonance in Medicine 2014;72(2):584-590.
4. Tropp J, Sugiura S. A dual-tuned probe and multiband receiver front end for X-nucleus spectroscopy with proton scout imaging in vivo. Magnetic Resonance in Medicine 1989;11(3):405-412.
5. Panda A, Jones S, Stark H, Raghavan RS, Sandrasegaran K, Bansal N, Dydak U. Phosphorus liver MRSI at 3 T using a novel dual-tuned eight-channel 31P/1H coil. Magnetic Resonance in Medicine 2012;68(5):1346-1356.
Figures