4109
Large field of view Sodium-23 and Carbon-13 imaging at 3T using a dedicated multinuclear birdcage body coil1Radiology, University of Cambridge, Cambridge, United Kingdom, 2Rapid Biomedical GmbH, Rimpar, Germany, 3MMIV, Radiology, Haukeland University Hospital, Bergen, Sweden, 4GE Healthcare, Munich, Germany
Synopsis
There has been a significant increase in carbon-13 and sodium-23 MRI research over the last decade, both in the areas of technical development and clinical applications. However, the lack of whole-body transmit systems for these nuclei limits large field of view imaging and uniform excitation. Here we demonstrate the imaging of natural abundance sodium and carbon signal from the abdomen using a 50 cm long 4-rung birdcage (40 cm inner bore) for abdominal 23Na/13C MRI. This whole body 23Na/13C coil has the potential to be used for a wide range of clinical applications.
Introduction
Sodium-23 (23Na) and carbon-13 (13C) magnetic resonance imaging (MRI) have been shown to have a wide range of clinical applications. There has been recent increased interest in multinuclear imaging with the advent of hyperpolarized technologies, such as 13C-MRI as a marker of metabolic activity(1). 23Na-MRI also indicates Na+K+ATPase activity in cancer and stroke(2-4), glycosaminoglycan changes in skin and cartilage (5-9), and absorption mechanisms in renal(10,11) and myocardial function(12). While multinuclear MRI has been investigated for several decades(13,14), its clinical potential remains unmet(15) due to the lack of appropriate whole-body radiofrequency (RF) coils.Heteronuclear MRI requires custom transmit RF coils, which are not integrated into clinical MRI systems. While there are several RF coil configurations use 23Na and 13C transmit/receive arrays, these often lack either transmit field uniformity or have a limited patient size that can be imaged. Here we demonstrate a 4-rung asymmetric birdcage for large field of view x-nuclear MRI, and perform 23Na and 13C imaging with the same hardware.
Methods
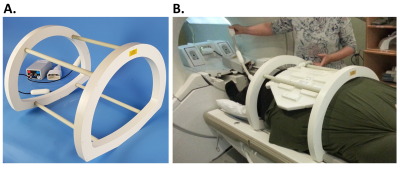
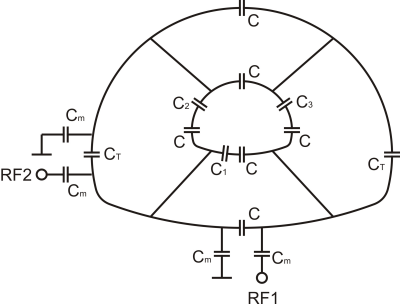
A custom transmit/receive 4-rung, 50cm long birdcage coil was developed (Figures 1&2; Rapid Biomedical GmbH, Rimpar, Germany), with rungs sited 30cm apart (equivalent to the clam-shell coil(16)). The coil was tuned and matched to 33.8MHz, which is the 23Na resonant frequency at 3T. The coil was adjusted to the table shape along the lower portion, with a rounded top that matched the MRI bore. The inner diameter of the end rings was 40cm diagonally; the coil outer diameter was 56cm at its maximal left-to-right length. The asymmetric shape required additional ring capacitors to balance the coil tuning. No proton traps were included into the coil for this developmental coil to simplify coil evaluation. A standard T/R switch with hybrid splitter was used for quadrature transmit/receive.Imaging was performed on a 3 T MRI system (MR750, GE Healthcare, Waukesha, WI). Sodium B1-maps were obtained with double angle mapping with a 60mM NaCl, 32x22x22cm3 phantom.
3D cones sodium images of a normal volunteer were also obtained with informed consent and ethical approval as follows: TR: 100ms, TE: 0.7ms, flip angle: 70°, voxel size: 4*4*8mm3, field-of-view: 48cm3, averages: 5, interleaves: 1402, bandwidth: 166kHz, total scan time: 11.7min. Matched 2D spiral images (5 slices, 6cm thick, 5mm spacing) were obtained of 13C and 23Na using the same 32-point k-space trajectory. The 13C was acquired with spectral-spatial excitation at the frequency of a natural abundance fat peak. We imaged a volunteer with a proton gradient echo sequence using a low flip angle to minimise patient heating, i.e., specific absorption rate (SAR).
Results
The |S11| of the coil at either I or Q port was 34dB and |S21| between ports was 24-26dB. The unloaded Q varied between 730-860 depending on port, which decreased to 23-27 when loaded with a large saline phantom.Figure 3 shows 23Na B1 maps, demonstrating that the B1 coefficient-of-variation was 16% across the entire phantom, other than near the rungs, where the B1 was nearly twice that of the nominal values.
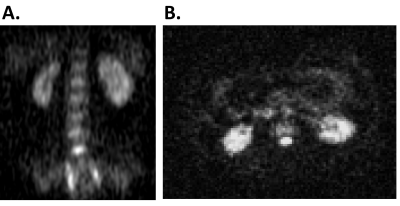
In vivo sodium MRI show the kidneys, liver, spleen, spine, and aorta (Figure 4). Figure 5 shows imaging of both 13C fat and 23Na. The small number of rungs enabled the possibility of imaging a large volunteer (Figure 1B). The 50cm length of the coil allowed 23Na imaging between the pelvis and heart, while still obtaining a 90° flip angle within a 1ms pulse width using an 8 kW power amplifier.
Discussion
We demonstrated 23Na and 13C body imaging with a large FOV, with a coil that can accommodate large patients. Future developments of the coil will enable a removeable top for easier patient positioning.This coil was built for 23Na, which is easier to test than 13C due to its higher single-peak in vivo signal. The coil was operable with sufficient SNR to obtain 13C fat images, as we have previously demonstrated in the prostate(17). The Q-ratio was very high, which occurs with large, low-frequency coils that are body-noise dominated. The high Q-ratio suggests that proton decoupling could be added without significant loss of efficiency, which would improve the safety for clinical 1H sequences. Our 13C results suggest that the coil could similarly be used at the 129Xe frequency without modification.
Despite these challenges, we achieved a 90˚ flip angle with a hard pulse width of 1 ms and <8 kW power. Transmit B1 power increases inversely with frequency, raising the difficulty of short pulses. The short T1 of 23Na (20-50ms) requires near 90˚ pulses for optimal SNR with TRs longer than 100ms, which increases SAR. The long pulse width results in a 4-fold linear increase of 23Na-SAR when compared with an equivalent 1H pulse with a 250µs pulse width, which allowed us to compare SAR with a 1H sequence. A birdcage such as this has quadrature transmission, which results in 40% decreased SAR when compared with a linear surface coil(18).
Conclusion
Here we demonstrate a 50cm long 4-rung 23Na/13C body coil with a very high field uniformity. This birdcage coil enabled imaging of the abdomen and has potential use with larger patients than is achievable with previous systems.Acknowledgements
This work has been supported by funding from GlaxoSmithKline, Cancer Research UK, European Union's Horizon 2020 research and innovation programme under grant agreement no. 761214, the National Institute of Health Research (NIHR) Cambridge Biomedical Research Centre and Addenbrooke’s Charitable Trust.References
1. Brindle KM, Bohndiek SE, Gallagher FA, Kettunen MI. Tumor imaging using hyperpolarized 13C magnetic resonance spectroscopy. Magnetic Resonance in Medicine 2011;66(2):505-519.
2. Ouwerkerk R, Bleich KB, Gillen JS, Pomper MG, Bottomley PA. Tissue sodium concentration in human brain tumors as measured with 23Na MR imaging. Radiology 2003;227(2):529-537.
3. Ouwerkerk R, Jacobs MA, Macura KJ, Wolff AC, Stearns V, Mezban SD, Khouri NF, Bluemke DA, Bottomley PA. Elevated tissue sodium concentration in malignant breast lesions detected with non-invasive 23Na MRI. Breast cancer research and treatment 2007;106(2):151-160.
4. Thulborn KR, Davis D, Snyder J, Yonas H, Kassam A. Sodium MR imaging of acute and subacute stroke for assessment of tissue viability. Neuroimaging Clin N Am 2005;15(3):639-653, xi-xii. 5. Borthakur A, Shapiro EM, Beers J, Kudchodkar S, Kneeland JB, Reddy R. Sensitivity of MRI to proteoglycan depletion in cartilage: comparison of sodium and proton MRI. Osteoarthr Cartilage 2000;8(4):288-293.
6. Reddy R, Insko EK, Noyszewski EA, Dandora R, Kneeland JB, Leigh JS. Sodium MRI of human articular cartilage in vivo. Magn Reson Med 1998;39(5):697-701.
7. Borthakur A, Shapiro EM, Akella SV, Gougoutas A, Kneeland JB, Reddy R. Quantifying sodium in the human wrist in vivo by using MR imaging. Radiology 2002;224(2):598-602.
8. Wheaton AJ, Borthakur A, Shapiro EM, Regatte RR, Akella SV, Kneeland JB, Reddy R. Proteoglycan loss in human knee cartilage: quantitation with sodium MR imaging--feasibility study. Radiology 2004;231(3):900-905.
9. Juras V, Zbýn S, Pressl C, Domayer SE, Hofstaetter JG, Mayerhoefer ME, Windhager R, Trattnig S. Sodium MR imaging of Achilles tendinopathy at 7 T: preliminary results. Radiology 2012;262(1):199-205.
10. Maril N, Rosen Y, Reynolds GH, Ivanishev A, Ngo L, Lenkinski RE. Sodium MRI of the human kidney at 3 Tesla. Magn Reson Med 2006;56(6):1229-1234.
11. Rosen Y, Lenkinski RE. Sodium MRI of a human transplanted kidney. Acad Radiol 2009;16(7):886-889.
12. Constantinides CD, Kraitchman DL, O'Brien KO, Boada FE, Gillen J, Bottomley PA. Noninvasive quantification of total sodium concentrations in acute reperfused myocardial infarction using 23Na MRI. Magn Reson Med 2001;46(6):1144-1151.
13. Ra JB, Hilal SK, Oh CH, Mun IK. In vivo magnetic resonance imaging of sodium in the human body. Magn Reson Med 1988;7(1):11-22.
14. Turski PA, Perman WH, Houston L, Winkler SS. Clinical and experimental sodium magnetic resonance imaging. Radiol Clin North Am 1988;26(4):861-871.
15. Steidle G, Graf H, Schick F. Sodium 3-D MRI of the human torso using a volume coil. Magn Reson Imaging 2004;22(2):171-180.
16. Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C] pyruvate. Science translational medicine 2013;5(198):198ra108-198ra108.
17. Barrett T, Riemer F, McLean MA, Kaggie JD, Robb F, Warren AY, Graves MJ, Gallagher FA. Molecular imaging of the prostate: Comparing total sodium concentration quantification in prostate cancer and normal tissue using dedicated 13C and 23Na endorectal coils. Journal of Magnetic Resonance Imaging 2019.
18. Tropp J, Calderon P, Carvajal L, Robb F, Larson PEZ, Shin P, Vigneron DB, Nelson SJ. A carbon receive array of 8 elements, interoperable with proton scanning, for human temporal lobe. Proc Intl Soc Mag Reson Med 2012;20.
Figures