4099
6-channel RF array for hyperpolarized 129Xe brain MRI at 3T1POLARIS,IICD department, University of Sheffield, Sheffield, United Kingdom
Synopsis
Dissolved phase hyperpolarised 129Xe is an emerging technique for perfusion imaging in the brain using inhaled gas as a perfusion tracer. As the dissolved 129Xe signal is inherently weak when compared to the gas phase signal, RF coil sensitivity is critical for high SNR 129Xe brain MRI. In this work, the design, development and testing of a novel 6 channel receiver and birdcage transmit coil RF system for dissolved phase 129Xe MRI in the human brain at 3T is demonstrated.
Introduction
Hyperpolarised 129Xe MRI when inhaled crosses the alveolar capillary barrier and dissolves in the red blood cells and plasma and its long T1 (~8s in oxygenated blood) allows its detection in other distal organs. Dissolved phase 129Xe spectroscopy and imaging in perfused organs such as kidneys and brain has been investigated1,2,3,4. In particular, the solubility of 129Xe in tissue itself shows clinical potential as a biomarker of tissue viability in stroke5 and blood brain barrier permeability. As the dissolved 129Xe signal is inherently weak when compared to the gas phase signal (~2%), RF coil sensitivity is critical for high SNR 129Xe brain MRI. This abstract describes the simulation of three receive array designs and the preliminary results of a complete transmit and receive setup for 129Xe human brain MRI at 3T.Material and Methods
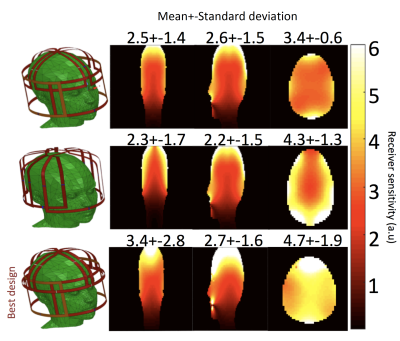
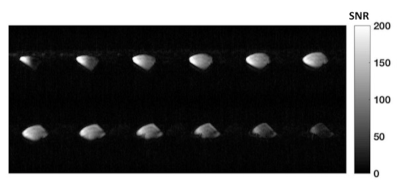
Transmit coil: is an 8-legs low pass birdcage with octagonal end rings. A capacitance of 28.6 pF was placed in each leg to tune the coil at 35.33 MHz (129Xe Larmor frequency at 3T). Two lattice baluns guarantee quadrature feeding of the coil. Active decoupling during the receive phase was achieved with high power PIN diodes, switched by DC trough an RF choke. Along the legs, proton traps enable the acquisition of proton images using the system Q-body coil (Acheiva, Philips). Receiver Array: Inspired by a previous work at 1.5T6, the array is built on an in-house 3D printed head former (Figure 1). The array consists of 6 elements matched to the available channels on the receiver chain. Preliminary simulations of three different designs were simulated in Ansys HFSS in order to evaluate the design with the highest intrinsic SNR. All single elements of the array were tuned at 35.33 MHz and fed with 1A current. The magnetic fields were then exported in MATLAB and combined to evaluate the array with the best sensitivity. The coil was then built in the workbench with high quality copper tubes and the circuit composes one-proton trap, a lattice balun for matching and a PIN diode for active decoupling with the transmit coil. The array was connected to the receive chain using λ/2 length cable to assure no change of phase in the input impedance for optimal preamp decoupling. Scanner experiments: All scans were performed on a 3T Acheiva Philips scanner. The coil was tested with a 129Xe gas phase phantom consisting of a bag hyperpolarized 129Xe that was imaged using a 3D spoiled gradient echo, TR/TE=6.7ms/2.5ms, acquisition matrix: 96x72x32, 8mm slice thickness, FA=10o, Receiver bandwidth=8.6 KHz. Before the scan, a flip angle calibration was performed: TR=2000ms; BW=4096 Hz, N of sample 256; 30 dynamics; nominal FA=120o. The decay of dynamic spectra of each element was then fitted according to , and the resulting FA was obtained from the average of all channels. Dynamic in vivo brain spectroscopy was acquired with only five channels. Scan was performed on a healthy male volunteer (27 years old, 75Kg), with a pulse acquire sequence: TR=2000ms, BW=4096 Hz, Number of sampling points 256 (spectra resolution of 16Hz/point), 25 dynamic scans; FA=16o; inhaled 129Xe Dose=1L (~30% of polarization), breathold time ~ 20 sec, total scan time ~ 50 sec.Results
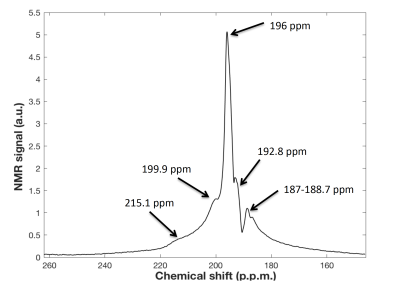
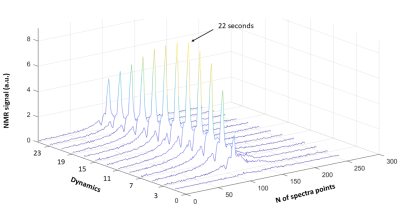
The transmit coil has an unloaded quality factor (Q) of 175 and a loaded Q of 61, resulting in a ratio Qload/Qunload of 2.89. The Results of EM simulations are shown in Figure 2. The optimum array design consists of two orthogonal saddle coil and 4 loops, which offer the best overall higher sensitivity along 3 planes and the best B1- field penetration along axial and sagittal planes. Phantom gas phase 129Xe images have a mean SNR~120 in the central slices. The average of the acquired dynamic spectra from the brain of the volunteer is shown in Figure 3 with the reference for all the peaks. Dynamic spectra show a maximum grey matter peak at 22 seconds (Figure 4).Conclusion
A novel RF setup for dissolved phase 129Xe brain MRI at 3T is presented consisting of a transmit birdcage and a 6-channel receiver RF coil. Trough preliminary EM simulation was found a design that guarantees an optimal coil sensitivity. The in vivo brain dissolved phase 129Xe spectra and 129Xe gas phantom images indicate high SNR for future dissolved 129Xe brain imaging.Acknowledgements
Acknowledgments: This work was funded by the Medical Research Council and the NIHR.References
1. Chacon‐Caldera, J, Maunder, A, Rao, M, et al. Dissolved hyperpolarized xenon‐129 MRI in human kidneys. Magn Reson Med. 2020; 83: 262– 270.
2. Rao, M., Stewart, N. J., Norquay, G., Griffiths, P. D. and Wild, J. M. (2016), High resolution spectroscopy and chemical shift imaging of hyperpolarized 129Xe dissolved in the human brain in vivo at 1.5 tesla. Magn. Reson. Med., 75: 2227-2234.
3. Hane, F.T.; Li, T.; Plata, J.-A.; Hassan, A.; Granberg, K.; Albert, M.S. Inhaled Xenon Washout as a Biomarker of Alzheimer’s Disease. Diagnostics 2018, 8(2):41.
4. Kilian, W., Seifert, F. and Rinneberg, H. (2004), Dynamic NMR spectroscopy of hyperpolarized 129Xe in human brain analyzed by an uptake model. Magn. Reson. Med., 51: 843-847.
5. Rao, M. R., Norquay, G., Stewart, N. J., Hoggard, N., Griffiths, P. D. and Wild, J. M. (2019), Assessment of brain perfusion using hyperpolarized 129Xe MRI in a subject with established stroke. J Magn Reson Imaging, 50: 1002-1004.
6. Imaging Human Brain Perfusion with Inhaled Hyperpolarized 129Xe MR Imaging; Madhwesha R. Rao, Neil J. Stewart, Paul D. Griffiths, Graham Norquay, and Jim M. Wild; Radiology 2018 286:2, 659-665
Figures

Figure 1: Overview of the 8-legs low pass birdcage with the 6-channel array built around the 3D printed former