4098
RF coil system for accelerated 129Xe hyperpolarized imaging at 1.5T1POLARIS,University of Sheffield, Sheffield, United Kingdom, 2Department of Medical Physics, Cross Cancer Institute, Edmonton, AB, Canada, 3GE Healthcare, Aurora, OH, United States
Synopsis
This abstract describes the development of a separate transmit/receive setup of coils for human chest 129Xe MRI at 1.5T. The transmit coil is an asymmetrical birdcage type designed to fit the scanner bore. The lumped components are calculated in consideration of the mutual coupling with the 1H body coil of the scanner, enabling the scanning of anatomical images and localization with the birdcage inside the bore. Furthermore, a parallel imaging strategy for 129Xe ventilation imaging has been tested to demonstrate accelerated acquisition times.
Introduction
Hyperpolarised 129Xe lung MRI is useful for the clinical assessment of many respiratory diseases such as asthma, Idiopathic Pulmonary Fibrosis and Chronic Obstructive Pulmonary Disease1. Imaging with 129Xe is typically performed during a single breath hold of the patient, therefore, parallel imaging can be a useful tool for shortening acquisition time. With a hyperpolarised sample there is the additional benefit that the reduced number of RF encoding pulses (higher acceleration factor) allows the use of a higher flip angle2, thus circumventing the SNR degradation typical of parallel imaging in 1H MRI. In this work, a custom RF coil system was developed for 129Xe lung MRI, consisting of a whole chest asymmetrical birdcage and an 8-channel receiver array. In advancement over previous designs3, coupling with the system’s 1H body coil was also considered in the design to ensure transparency to 1H RF, enabling easy anatomical imaging of the lungs and co-registration with 129Xe ventilation images.Material and Methods
RF setup: The topology of the birdcage was optimised for patient comfort and to make full use of the magnet bore, in conjunction with providing a homogeneous B1+ transmit field over the whole chest. The geometry is the same as described in previous work for 3He lung MRI4,5, and consists of a 12-leg asymmetrical band pass type birdcage coil (Diameter=55cm; Height=40cm) as shown in (Figure 1a). In order to produce a homogeneous RF field, a conformal mapping method combined with an algebraic method was used to calculate the capacitances at each element of the coil’s ladder network. To tune the coil for operation inside the bore of the magnet, measurements of the mesh element’s self and mutual inductances first was done using two-port isolation S21 measurements4. The mechanical support of the structure is a frame made from a glass reinforced and flame retardant fiberglass, and to facilitate positioning of the patient, the whole structure can be split in two halves along the coronal plane with four nonmagnetic beryllium copper rods at the corners for connection. Printed circuit boards were soldered along the legs and end rings to allow for the placement of tuning capacitors. The receiver array (Figure 1b), is an ex-proton 8 channel cardiac array coil (GE) that was retro tuned to work at the 129Xe frequency (Figure 1b). Both coils are tuned to work at the 129Xe Larmor frequency at 1.5T (17.66 MHz). Scanner experiments: 129Xe ventilation imaging was performed with a 3D Steady State Free Precession (SSFP) sequence with parameters: TR/TE=6.5/3.1ms,10 mm coronal slices, field of view 40x40cm2, acquisition matrix of 80x80x20, receiver bandwidth (BW) of ±5.95 kHz. For accelerated acquisitions, under sampling was performed along the left-right phase-encoding direction. In order to have the same k-space filter for each acceleration factor (R=1,2,4), the optimal SSFP flip angles (α) was calculated using the simulation formula described in6. All experiments were performed on a 1.5T GE HDx scanner in a healthy male volunteer (29 years old, 70kg), and for each scan, a gas mixture of 500 ml of 129Xe (~30% of polarization) and 500 ml of N2 was inhaled. The centre of k-space was fully sampled with an auto calibration region (ACS) of 20x20 k-space lines, respectively along the phase and the slice encoding directions. Before ventilation scans, anatomical proton images of the lungs were acquired (3D Coronal SPGR matrix 80x80x20, BW=83KHz; FOV=40x40 cm2, TR/TE=1.5ms/0.6ms, 5 Averages). Images reconstruction: Sensitivity maps were obtained through the filtering of the autocalibration region with a Kaiser window to attenuate the higher frequencies5. The cropped k-space was then zero-filled to the full encoded matrix size and Fourier transformed. Finally, 2DSENSE was used to reconstruct the aliased images7.Results
Optimal flip angles from SSFP simulation were 10.3o,14.1o,17.5o respectively for full sampled, R=2 (1000 RF pulses), and R=4 (700 RF pulses) (Figure 2). Total scan times were 11s, 8s and 6s for R=1, 2, 4 respectively. 1H images acquired in-situ show an optimal co-registration with the 129Xe images (Figure 3). Coronal and axial lung images with respective g-factor maps are shown in Figure 3. Image reconstruction for R=2 (Figure 4) shows a mean SNR of 150 in the central slices with no visible artefacts. A decrease of image quality is noticeable for images with an acceleration factor of R=4 (Figure 5), where also some aliasing artefacts are still visible in the posterior slices (local g-factor>3).Conclusion
A whole chest RF setup for 129Xe has been successfully built allowing faster acquisition of ventilation images through parallel imaging, where an acceptable image quality was observed with an acceleration factor of R=2. In advance of previous elliptical birdcage designs for 3He and 129Xe, the design and tuning of the birdcage in the absence of an RF shield allow acquisition of coregistered proton images in the same scan session as the 129Xe images without moving the subject.Acknowledgements
This work was funded by the Medical Research Council.References
1. Mugler III J. P., Altes T.A. Hyperpolarized 129Xe MRI of the human lung, JMRI 37:313–331 (2013).
2. Lee R.F. et al, Advantages of Parallel Imaging in Conjunction with Hyperpolarized Helium—A New Approach to MRI of the Lung, Mag. Res. Med. 55:1132– 1141 (2006).
3. Dregerly I. et al., 32-Channel Phased-Array Receive with Asymmetric Birdcage Transmit Coil for Hyperpolarized Xenon-129 Lung Imaging ,Mag. Res. Med. 70:576–583 (2013).
4. De Zanche N., et al. Asymmetric Quadrature Split Birdcage Coil for Hyperpolarized 3He Lung MRI at 1.5T, Mag. Res. Med. Magnetic Resonance in Medicine 60:431–438 (2008).
5. Deppe M. H. et al., A flexible 32‐channel receive array combined with a homogeneous transmit coil for human lung imaging with hyperpolarized 3He at 1.5 T, Mag. Res. Med. 66:1788–1797 (2011).
6. Wild J.M. et al., Steady-state free precession with hyperpolarized 3He: Experiments and theory, Mag. Res. Med. 60:431– 438 (2008).
7. Weiger M. et al., 2D SENSE for faster 3D MRI, MAGMA 14:10-19 (2002).
Figures

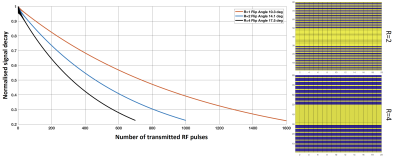
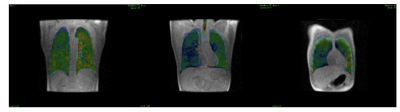
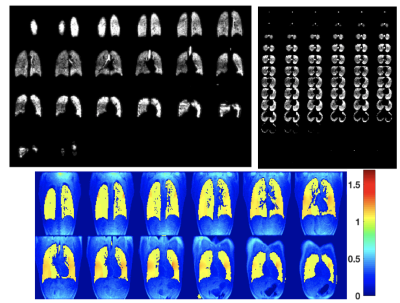
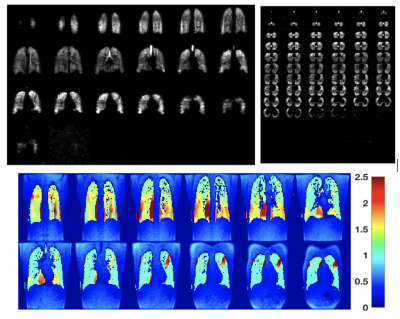
Figure 4: Coronal and axial reconstruction of 3D ventilation images for R=2;
below are shown g-factor maps in the central coronal slices