4083
Improved Brain Imaging with an Integrated High-Permittivity Material Head Array1Bernard and Irene Schwartz Center for Biomedical Imaging, Department of Radiology, NYU School of Medicine, New york, NY, United States, 2Center for Advanced Imaging Innovation and Research (CAI2R), Department of Radiology, NYU School of Medicine, New york, NY, United States, 3HyQRS LLC, State College, PA, United States, 4The Sackler Institute of Graduate Biomedical Science, New york, NY, United States
Synopsis
High electric permittivity materials (HPM) have been used to improve transmit efficiency (B1+) and receive sensitivity in targeted regions. In this work we demonstrate improvements in the entire brain by designing and implementing an integrated HPM shell for a head coil with 8-channel transmit and 30-channel receive arrays. The coil with HPM insert provides gains of approximately 55% in transmit efficiency and 20% in SNR compared to an identical coil without the insert.
Introduction
Studies have shown that excitation efficiency (B1+), homogeneity and signal to noise ratio (SNR) can be improved in specific anatomical areas by strategically locating high electric permittivity materials (HPM) 1-5. Usually in these demonstrations the tissue volume impacted by the HPM is relatively small compared to coil coverage. Conversely the effect of a HPM insert that encompasses the entire imaging volume of a receive array is a relatively nascent topic with limited reports6. Our preliminary simulation study showed evidence that an HPM insert encompassing the head provides significant B1+ and SNR gains in the entire brain at 7T7. This motivated us to determine whether the simulated performance could be realized in practice. To do so, we manufactured a custom HPM helmet with integrated 30-channel receive and 8-channel transmit arrays and compared its performance to an identical array without the helmetMethods
HPM and Coil Construction: To investigate the benefit of a fully surrounding HPM insert on 7T coil performance, two 30-channel receive arrays were built on identical 3D printed polycarbonate substrates (Fortus 360mc, Stratasys, Inc. 21 x 32 x 27 cm). The “HPM array” was integrated with a custom ceramic HPM helmet (HyQRS, LLC, εr = 107, σ = 0.004 S/m, thickness = 8mm) that fit tightly into the substrate (Fig.1). For direct comparison, we built an “empty shell (ES) array” that enclosed a hollow plastic helmet (εr ~ 1, σ ~ 0) whose geometry was identical to the HPM helmet (Fig 2). We built the receive arrays with a gapped design (Fig.1, 2) known to perform well at 7T8. The arrays contain eight rows of coils. The coils were tuned and matched in the presence of the HPM helmet and a tissue-equivalent head phantom9 for the HPM array, and the plastic helmet and head phantom for the ES array. Neighbor coils ( H-F direction) were geometrically decoupled, while preamplifier decoupling was used to isolate other combinations. One active detuning circuit and one current fuse (700mA) were incorporated per coil in both arrays to ensure safety. Excitation was produced by an 8-channel dipole array10 that surrounded the receive array (Fig.1, 2). The dipoles were tuned and matched in the presence of the receive array and head phantom and did not require retuning in the presence of the HPM helmet.Imaging: Measurements were performed on a whole-body 7 T scanner (MAGNETOM, Siemens, Healthineers, Germany) with eight independent transmit amplifiers. Spin excitation was performed using B1+ shimming wherein the pulse phases were adjusted for each experiment to provide constructive interference in the center of the brain. All in vivo experiments were performed upon approval by our local IRB and with informed written consent from the participants. B1+ and SNR maps were acquired using TurboFLASH11 (TE/TR-1.9/10000ms) and GRE acquisitions (with and without RF).
Results
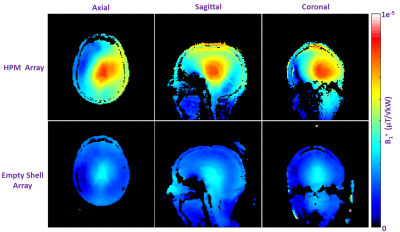
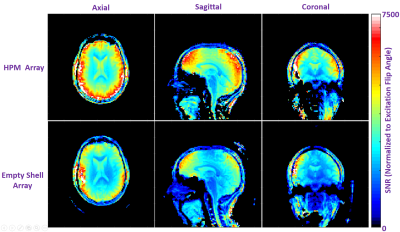
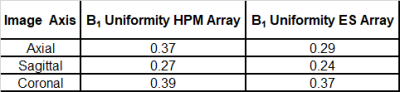
Bench Measurements: The receive coil unloaded Q, loaded Q, and capacitance required for resonance were 245, 65 (ratio = 3.8), and 6.8 pF in the ES array, and 70, 15 (ratio = 4.7), and 5.6 pF in the HPM array. We believe the HPM shell effectively increased the coil dimensions due to expanded, distributed displacement current pattern in the HPM, which may have contributed to reduced unloaded Q (possibly due to increased radiation losses), improved Q ratio (due to increased coil to tissue coupling), and increased effective inductance. All HPM and ES coils were easily tuned and matched; the average reflection (S11) was approximately -19 dB for the HPM and ES elements. The average isolation (S12) between overlapped neighbor elements was -11.1 dB and -11.7 dB respectively for the HPM and ES elements. The HPM shell caused minimal changes in overlap required for geomteric decoupling.Imaging:The HPM helmet improved B1+ efficiency by 56% over the empty shell at the center of the brain (Figure 3). The HPM array setup required a 500μs, 72.2v hard pulse to achieve 90° flip angle compared to 121.5v, 500μs hard pulse for the ES. The B1+ uniformity in the cerebrum is summarized in Table 1. Normalized SNR maps are presented in Figure 4. SNR in the whole cerebrum in the axial slice was 21% better for the HPM array compared to the ES array. Similar boosts are observed in sagittal and coronal SNR maps.
Conclusions and Discussion
We designed and implemented a 30-channel receive array with an integrated volumetric HPM shell to demonstrate B1+ and SNR improvement in the brain. Our encompassing HPM shell provided an average of 21% SNR and 55% B1+ improvement in the entire cerebrum. While our transmit setup was large (~33cm. dia) and by no means optimal, it is noteworthy that the HPM helmet substantially focused B1+ in the brain volume. On the receive side, we did not achieve as high an SNR boost as predicted by our simulations in the center of the brain, which might be due to limited realism in simulations regarding coil geometry, coil losses, and/or radiation losses. Importantly, significant improvements were seen in the SNR at the center of the brain for both simulations and experiment, which is challenging to achieve by other methods12. In summary volumetric HPM shells can be used in a RF coil to maximize transmit efficiency and SNR.Acknowledgements
The authors thank Bei Zhang and Martijn Cloos for their contributions on the transmit array setup.References
1. Alsop et al., MRM 1998; 40:49-54.
2. Yang et al., JMRI 2006; 24:197-202.
3. Haines at al., JMRI 2010; 203:323-327.
4. Webb, Con in MR pt. A 2011; 38(4):148-84.
5. Sica et al., 2019 ISMRM, p. 569.
6. Collins et al., 2014 ISMRM, p.404.
7. Carluccio et al., ISMRM p. 4405.
8. Wang et al., Phys. Med Biol, 56:2779-2789.
9. Duan et al., Medical Physics, 2014; 41(10):1023.
10. Zhang et al., 2016 ISMRM p. 3508.
11. Fautz et al., 2008 ISMRM, p.1247.
12. Wiggins et al., MRM 2009; 62(3):754-62.
Figures