4068
Flexible Self-decoupled Dual-tuned Array1Department of Radiology, Vanderbilt University Medical Center, Nashville, TN, United States, 2Institute of Imaging Science, Vanderbilt University, Nashville, TN, United States
Synopsis
Highly flexible or wearable dual-tuned coil arrays appear to be good choices for increasing the SNR for both protons and X-nuclei and for improving patient comfort. However, complex couplings arise in a dual-tuned coil array. In this work, we have designed self-decoupled coils within a flexible, nested, dual-tuned array, in which impedances are redistributed to achieve high inter-element isolation.
Purpose
A recurring need in MRI and MRS is to acquire high-quality signals from multiple nuclei in the same study. Highly flexible or wearable dual-tuned coil arrays appear to be good choices for increasing the SNR for both protons and X-nuclei and for improving patient comfort. However, complex couplings arise in a dual-tuned coil array: these include the crosstalk between proton coils in adjacent elements; the crosstalk between X-nuclei of adjacent elements; and the crosstalk between the proton and X-nuclear coils in the same element. When expanding a nested dual tuned coil [1-5] to array designs, suitable decoupling strategies have to be used for both proton and X-nuclei arrays. A straightforward method is to overlap adjacent elements to minimize inductive couplings. However, the overlap area cannot be optimized for both nuclei simultaneously. For instance, when the X-nucleus coils overlap by ~10%, the proton coils overlap with a much smaller area (Figure 1). Although capacitive or inductive decoupling circuits for the proton array can be added, this requires the use of lumped/rigid capacitor/inductors that become obstacles to making a highly flexible coil. We have designed self-decoupled coils within a flexible, nested, dual-tuned array, in which impedances are redistributed to achieve high inter-element isolation.Methods
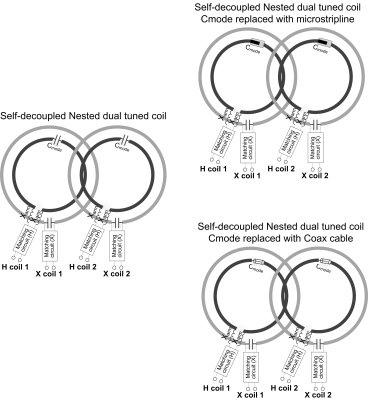
Figure 2a shows the diagram of a 2-element nested dual-tuned coil array. In each, the X-nucleus coil (gray color in Figure 2a) is positioned as the outer ring and the proton coil (black color in Figure 2a) is positioned as the inner ring. Adjacent outer rings are overlapped to a critical area (~10%) to minimize the inductive coupling of two X-nuclei coils. Cmode in the proton coils is tuned to balance the loop and dipole modes and make the magnetic coupling coefficient (Km) and the electric coupling coefficient (Ke) meet the decoupling condition: Km+Ke=0 [6]. To make the coil more flexible and durable, the lumped element Xarm is integrated into the feeding board and the lumped element Cmode can be replaced with an equivalent microstrip capacitor (Figure 2b) and an equivalent coaxial capacitor (Figure 2c).We calculate the scattering parameters and electromagnetic fields of self-decoupled dual-tuned arrays for 3T and 7T 1H/23Na imaging with commercial simulation software (Ansys HFSS, Canonsburg, PA, USA). The diameter of the concentric proton coil and sodium coil is 7 cm and 10 cm respectively. In all simulations, coils are well-matched, tuned and decoupled as for real cases. Meandering lines are used for 3T proton coils to avoid the need for large inductances (Xarm). Based on the simulation results, we fabricated and bench-tested a 2-element dual-tuned array for 7T proton/sodium (1H/23Na) imaging.
Results
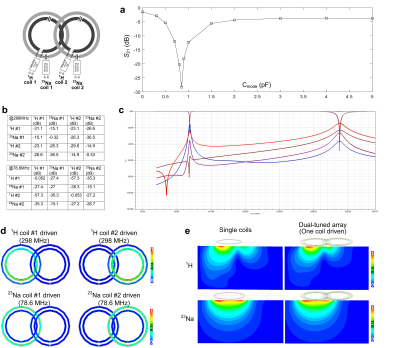
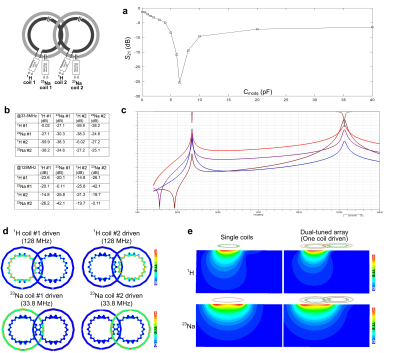
Figure 3a shows the simulated transmission coefficients (S21) of two adjacent proton coils for different values of Cmode. The isolation is small (<-25 dB, <1% power cross-talk) when Cmode = 0.85 pF at 7T and 6.5 pF at 3T. Note that two proton coils will strongly couple to each if Cmode is not optimized (-3.8 dB at 3T and -6.3 dB at 7T). Figure 3b shows the S-parameter values at the Larmor frequencies of 1H and 23Na, and Figure 3c shows a plot of the S-parameters versus frequency. The coupling between adjacent 1H coils, and between adjacent 23Na coils is -23.1 dB and -15.1 dB, respectively. The worst coupling between proton coil and 23Na coil occurs within the same element, but it still can achieve -27.4 dB at 78.6 MHz and -15.1 dB at 298 MHz. Figure 3d shows the B1 fields from one coil of the dual-tuned array (other coils terminated with 50 ohm) and its comparison with a single-coil (without the presence of other coils). It is found that there is almost no B1 efficiency losses compared to ideal single coils, which is also consistent with the S-parameter results.Figure 4 shows the simulation results at 3T, where the Larmor frequencies of 1H and 23Na are 128 MHz and 33.8 MHz, respectively. Similar to the 7T dual-tuned coil, 3T coils exhibit acceptable isolation and almost no B1 efficiency decreases. It may also be noted that the required Xarm is only 30 nH when using meander lines.
Conclusion
We extend the self-decoupled coil concept to design flexible dual-tuned arrays. Although we demonstrate the application of self-decoupling technology only to nested dual-tuned arrays, it is also applicable for other types of dual-tuned coils, such as dual-layer and common mode/differential mode coils [7-9]. The simulation and bench test results show that all coils are highly decoupled and thus have almost the same B1 properties (i.e., transmit efficiency and SNR) as ideal single mononuclear coils. All lumped elements are either integrated into the feeding circuit or are replaced with transmission lines and thus the coil is highly flexible and even foldable. Note that this approach can in principle be applied to both transmit/receive as well as receive-only arrays.Acknowledgements
No acknowledgement found.References
1. Brown, R., Madelin, G., Lattanzi, R., Chang, G., Regatte, R. R., Sodickson, D. K., & Wiggins, G. C. (2013). Design of a nested eight‐channel sodium and four‐channel proton coil for 7T knee imaging. Magnetic resonance in medicine, 70(1), 259-268.
2. Fitzsimmons, J. R., Brooker, H. R., & Beck, B. (1987). A transformer‐coupled double‐resonant probe for NMR imaging and spectroscopy. Magnetic resonance in medicine, 5(5), 471-477.
3. Fitzsimmons, J. R., Brooker, H. R., & Beck, B. (1989). A comparison of double‐tuned surface coils. Magnetic resonance in medicine, 10(3), 302-309.
4. Mispelter, J., Tiffon, B., Quiniou, E., & Lhoste, J. M. (1989). Optimization of 13C-{1H} double coplanar surface-coil design for the WALTZ-16 decoupling sequence. Journal of magnetic resonance, 82, 622-628.
5. Brown, R., Lakshmanan, K., Madelin, G., & Parasoglou, P. (2016). A nested phosphorus and proton coil array for brain magnetic resonance imaging and spectroscopy. Neuroimage, 124, 602-611.
6. Yan, X., Gore, J. C., & Grissom, W. A. (2018). Self-decoupled radiofrequency coils for magnetic resonance imaging. Nature communications, 9(1), 3481.
7. Kim, J. H., Moon, C. H., Park, B. W., Furlan, A., Zhao, T., & Bae, K. T. (2012). Multichannel transceiver dual-tuned RF coil for proton/sodium MR imaging of knee cartilage at 3 T. Magnetic resonance imaging, 30(4), 562-571.
8. Cao, P., Zhang, X., Park, I., Najac, C., Nelson, S. J., Ronen, S., & Larson, P. E. (2016). 1H‐13C independently tuned radiofrequency surface coil applied for in vivo hyperpolarized MRI. Magnetic resonance in medicine, 76(5), 1612-1620.
9. Goluch, S., Frass-Kriegl, R., Meyerspeer, M., Pichler, M., Sieg, J., Gajdošík, M., ... & Laistler, E. (2018). Proton-decoupled carbon magnetic resonance spectroscopy in human calf muscles at 7 T using a multi-channel radiofrequency coil. Scientific reports, 8(1), 6211.
Figures