4061
A Dedicated 17O Rx Array to Assess Renal Metabolism of Donor Kidneys1Dept. of Radiology, Medical Physics, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 2German Consortium for Translational Cancer Research Partner Site Freiburg, German Cancer Research Center (DKFZ), Heidelberg, Germany, 3NUKEM Isotopes GmbH, Alzenau, Germany, 4Department of Surgery – Organ Donation and Transplantation, University Medical Center Groningen, Groningen, Netherlands, 5Medical Imaging Center, University Medical Center Groningen, Groningen, Netherlands
Synopsis
Direct 17O-MRI can be used to measure renal metabolism in perfused kidneys in an organ transplantation setup. To optimize SNR, a dedicated 17O Rx array was designed that fits into a perfusion box used for functional metabolism tests of the donor kidneys. The increased filling ratio resulted in higher SNR compared to the volume and surface Tx/Rx coils. In combination with a 17O birdcage Tx coil for homogeneous excitation the 4-element Rx array could also be used for parallel imaging.
Introduction
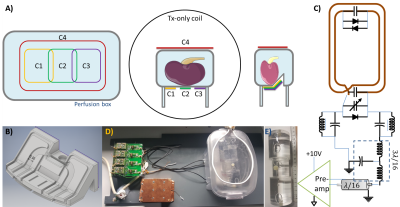
Most organ transplants are renal transplants [1–3], but even though biomarkers exist to diagnose complications after kidney transplantation [4], survival rates decrease drastically with the time after transplantation [5,6]. One reason for post-transplantation complications is the insufficient functional characterization of the transplanted kidney. We have shown previously that, in addition to perfusion and renal filtration, tissue oxygenation of a resected kidney can be quantified in vitro before implantation using dynamic 17O MRI [7].A homogeneous excitation field (B1) and high SNR are important for high-resolution 17O MRI of the kidney, which in whole body 1H MRI is achieved by combining a local Rx array with a large volume Tx coil. In this work, we designed and constructed a 17O RF coil array that fits on a sterilized perfusion box used for functional metabolism tests of the donor kidneys which was combined with a 17O birdcage head coil for homogeneous RF excitation.
Methods
Rx ArrayA schematic of the coil arrangement is shown in Figure 1A. Three of the 4 Rx elements (C1-C3) were mounted on a 3D-printed holder (Fig.1B) that fits under the perfusion box in which the kidney is stored between resection and transplantation. C1-C3 were constructed using two turns of a tin-plated copper wire (∅=1mm) on a rectangular shaped former of 3x5cm2 size. The tuning capacity was distributed on two elements, one of which was used as the input port for tuning and matching (Fig.1C). A larger (∅=15cm) Rx element (C4) was placed on top of the perfusion box using a single turn (∅=5mm) of copper tube (Fig.1D). C2-4 were geometrically decoupled, and all elements were further isolated from each other by preamplifier decoupling [8]. A separate custom-made quadrature-driven 4-leg birdcage volume coil was used as the Tx only element. Measurement of the reflection and transmission coefficients, Sij, were performed on a vector network analyzer (ZVB 4; Rohde&Schwarz, Munich Germany) for each channel pair with all other channels terminated with 50Ω. Noise correlation matrix of the Rx array was also measured using a FID sequence (TR/TE 200 ms/0.56 ms, BW 10 kHz, Peak Tx RF voltage 0V) [9].
MRI Measurements
A cylindrical water phantom composed of three separate compartments was used to test performance of the coil array at a clinical 3T MR system (Prisma; SIEMENS, Erlangen, Germany). The inner cylinder (modeling the medulla) was filled with 17O2-enriched saline solution (c17O2 = 0.072%) and has an inner diameter of 3 cm and a length of 4 cm. In total, volume (V = 247mL) and axial dimensions are similar to those of a human kidney [10]. For image acquisition a radial 3D UTE sequence with golden-angle projection acquisition pattern was used [11]. Kaiser-Bessel-regridding [12] of k-space data and Hanning-filtering was subsequently applied. For comparison, a custom built Tx/Rx head coil, and a loop coil were also used to acquire 17O MRI data using the same protocol (TR/TE 6ms/0.52ms, FA π/6, BW 390 Hz/px, radial spokes 80000). The SNR of a measurement was quantified according to [13].
Results
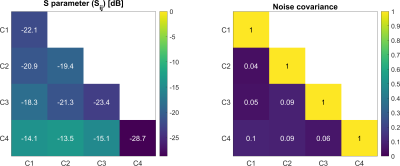
S-parameter and noise correlation matrices are shown Figure 2. The range of coupling between overlapping/non neighboring elements was 13.5-21.3 dB/ 13.5-18.3 dB, to which preamplifier decoupling (75cm-long coaxial line and lumped element 3l/16 tank circuits between coil and preamplifier interface) added another 13 dB. An average unloaded/loaded quality factor of QU = 150.3 / QL = 80.2 was measured resulting in a Q-ratio of 1.87. The active detuning efficiency was measured as 38+-3 dB for all elements. Noise correlation values ranged from 4% to 10% (Fig.2). The reconstructed images of the kidney phantom are shown in Figure 3 as cross-sections from different axes. The SNR values of the center/left/right section of the phantom are 98/66/81 for single loop coil, 97/65/70 for the birdcage coil, and 481/425/404 for the 17O Rx array, respectively, which is an up to 5-fold increase in SNR using the Rx array.Discussion
As expected, the SNR of the central cylinder was higher for all coils due to the addition of H217O. The performance of the Rx array was superior to single channel Tx/Rx loop and birdcage coils due to the smaller coil element size, and the higher filling ratio. Single loop coils, however, are not preferable for quantitative imaging tasks due to an SNR difference of 19% between left and right cylinders, in the SNR comparison. C4 is placed on top of the perfusion box, therefore has higher coupling with C1-3. Since there is a distance of at least 8 cm between the center of the kidney and the loop coil, fields of a smaller loop coil would not be able to penetrate deep enough to reach the kidney. In a next step the increased image quality of the coil array will be used to increase the spatial and temporal resolution in dynamic 17O MRI measurements in kidneys to identify e.g. induced ischemic lesions.Acknowledgements
Support from NUKEM Isotopes Imaging GmbH is gratefully acknowledged.References
1. Branger, P. & Samuel, U. Annual Report 2017. 124 (Eurotransplant International Foundation, 2017).
2. Hart, A. et al. OPTN/SRTR 2016 Annual Data Report: Kidney. Am. J. Transplant. 18, 97.
3. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation. Annual Report of theU.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: UNOS (2016). Available at: https://unos.org/about/annual-report/2016-annual-report/. (Accessed: 30th October 2018)
4. Salvadori, M. & Tsalouchos, A. Biomarkers in renal transplantation: An updated review. World J. Transplant. 7, 161–178 (2017).
5. Pestana, J. M. Clinical outcomes of 11,436 kidney transplants performed in a single center - Hospital do Rim. J. Bras. Nefrol. 39, (2017).
6. Wang, J. H., Skeans, M. A. & Israni, A. K. Current Status of Kidney Transplant Outcomes: Dying to Survive. Adv. Chronic Kidney Dis. 23, 281–286 (2016).
7. Taege, Y., Fischer, J., Özen, A. C., et al. Protocol Optimization for Functional 17O-MRI of Donor Kidneys at 3T. In Proc. 27th ISMRM, Montreal, QC, Canada. p. 4223.
8. Roemer, P.B., Edelstein, W.A., Hayes, C.E., Souza, S.P., Mueller, O.M. The NMR phased array, Magnetic Resonance inMedicine 16, 192–225 (1990).
9. Kellman, P., & McVeigh, E. R. Image reconstruction in SNR units: a general method for SNR measurement. Magnetic resonance in medicine, 54(6), 1439–1447 (2005).
10. Cheong, B. et al. Normal values for renal length and volume as measured by magneticresonance imaging. Clinical Journal of the American Society of Nephrology 2.1 (2007).
11. Chan, R. W., Ramsay, E. A., Cunningham, C. H. & Plewes, D. B. Temporal stability of adaptive 3D radial MRI using multidimensional golden means. Magn. Reson. Med.61, 354–363 (2009).
12. Jackson, J. I., Meyer, C. H., Nishimura, D. G. & Macovski, A. Selection of a convolution function for Fourier inversion using gridding (computerised tomography application).IEEE Trans. Med. Imaging 10, 473–478 (1991).
13. Gudbjartsson, H. H. and Patz, S. The Rician
Distribution of Noisy MRI Data. Magnetic Resonance in Medicine 34.6 (1995).
Figures