4038
Validation and Safety Approval of a Dual-Mode Head Coil for pTx Applications In Vivo at 7 Tesla1Imaging Centre of Excellence, University of Glasgow, Glasgow, United Kingdom, 2MRI Physics, NHS Greater Glasgow and Clyde, Glasgow, United Kingdom, 3Imaging Centre of Excellence, Siemens Healthcare Ltd. United Kingdom, Glasgow, United Kingdom, 4MR CoilTech, Glasgow, United Kingdom
Synopsis
Following regulatory approval of single-transmit MRI at 7 tesla, there is a rapidly growing interest in clinical MRI at this field strength. However, the wider use of diagnostic MRI at 7T will require imaging in parallel-transmit (pTx) mode to reduce B1+ inhomogeneity. Previous work introduced a dual-mode head coil that operates in both transmit modes and this study investigates the use of this coil for the pTx case. It also describes the safety procedure that was followed to ensure safe operation for human scanning using the real-time SAR supervision on a commercial scanner.
Introduction
The higher spatial resolution provided by 7T MRI of the brain leads to improved diagnostic confidence compared to scanning at 3T [1] and there is an increasing interest in clinical applications. Regulatory approval has been granted in the USA and EU for clinical 7T imaging in single-transmit (1Tx) mode, but this is not yet the case for parallel-transmit (pTx) methods [2], which overcome the poor B1+ homogeneity at higher field strengths [3]. The current generation of commercial 7T systems support both modes of operation, but require separate RF coils in each case. Previous work [4] addressed this issue, by introducing an open-face, dual-mode coil design that can be used for both 1Tx and pTx data acquisition. This study describes the initial application of this coil type for pTx scanning in vivo, including details about the safety approval process used to address the complex SAR considerations required for pTx operation.Methods
The dual-mode head coil consists of 8 transmit and 32 receive elements, and interfaces with both 1Tx and pTx modes of the 7T scanner (MAGNETOM Terra, Siemens Healthcare, Erlangen, Germany). The coil is locally shielded and designed with large eye cutouts to provide an open feel for patient comfort by optimizing the design in CST Studio Suite (Dassault Systemes) [4,5]. Extensive validation was required for local ethics committee approval in pTx mode, as the superimposed RF pulses can generate locally elevated SAR values that are several times greater than the average whole-head value [6].Following a similar heating experiment as Hoffman et al. [7], a sugar-salt-agar phantom was heated for 17min in two experiments using different transmit modes: circularly polarized (CP) with 23.1W, and a second phase configuration with 21.9W (amplitudes equal across channels). The phase images of a gradient echo scan before and after heating were used to estimate temperature change within the phantom [8] and were corroborated with a CST simulation.
The fidelity of each transmit channel was validated by comparing experimental and simulated single-channel B1+ maps. Experimental maps were collected in a head-and-shoulders phantom with a presaturation TurboFLASH sequence [9].
The SAR monitoring on the 7T Terra is implemented by virtual observation points (VOPs) [6]. These were generated with an overestimation of 10% for our coil by concatenating three 1mm isotropic body model simulations (Duke and Ella from the Virtual Family cohort [10] and Gustav from CST), each at three longitudinal positions. By design, VOPs will always meet or exceed full-body model SAR estimates, and maximum SAR reported by the scanner will be even more conservative. To test this relationship, the CST- and VOP-based maximum 10g SAR values were estimated for fixed input power and three pTx modes: CP, CP2+ (fixed amplitude and 90° phase between channels), and the 'worst case' excitation vector generated during VOP compression. These values were compared to the scanner-reported SAR for the same power.
After above tests, the coil was approved by local safety and ethics committees for scanning healthy subjects in pTx mode. To test the functionality of pTx, a consented volunteer was scanned with the TurboFLASH B1+ mapping protocol (single channel and combined modes), as well as a T1-weighted FLASH and T2-weighted TSE. A CP-mode configuration was compared to an optimized flip angle (FA) magnitude least-squares shim [11] with constraints on flip angle standard deviation and SAR.
Results
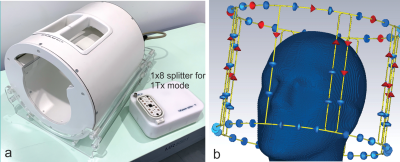
Figure 1 shows our dual-mode coil and the corresponding CST simulation of the coil and a home-built head-and-shoulders phantom.Figure 2 compares simulated and measured temperature maps from the heating tests conducted in the sugar-salt-agar gel phantom. The distribution and temperature rises between simulation and measured data are in good agreement, with differences attributed to the time-scales of CST (instantaneous power) vs. experiment (power deposition over 17min).
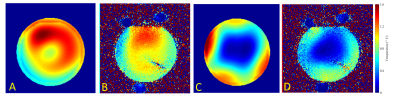
Figure 3 shows simulated and experimental complex B1+ maps for the head-and-shoulders phantom. After subtracting the phase from a set reference coil, both magnitude and phase maps are in excellent agreement.
Figure 4 contains two tables. Table A reviews maximum local SAR estimations from the CST model, VOP compression, and reported scanner values. As desired, the scanner is the most conservative. Table B reports flip angle mean, standard deviation, input power, and SAR for the scan performed in vivo, comparing CP and B1+ shim modes. An added benefit of the pTx mode is that it had lower local SAR than conventional CP.
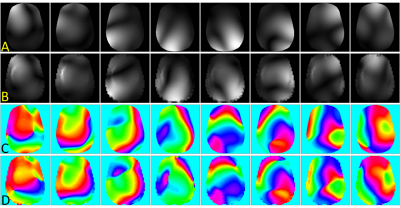
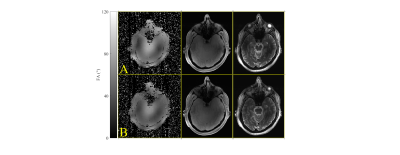
Figure 5 shows the pTx coil results for a CP mode acquisition and B1 shims optimized to the volunteer B1+ map. In both the FLASH and TSE images there is significant improvement in the left temporal lobe signal that was lost to B1+ inhomogeneity.
Discussion and Conclusion
Although 7T MRI can improve diagnostic confidence, wider clinical application is limited by B1+ inhomogeneity. This strongly motivates to integrate pTx methods into routine examinations and to explore optimized coil designs that suit clinical requirements. The coil used in this study provides clinical benefit by avoiding the closed design used by other 7T head coils and by operating in both 1Tx and pTx modes. The coil improved image uniformity using B1 shimming and passed a rigorous safety approval procedure, verifying that real-time scanner supervision keeps local SAR values within safe limits.Acknowledgements
Patrick Liebig, Rene Gumbrecht, Natasha Fullerton, Tracey Hopkins, Rosemary Woodward, Graeme Keith
References
[1] Springer et al., “Comparison of routine brain imaging at 3 T and 7 T,” Invest. Radiol., 2016.
[2] T. Ibrahim et al., “Application of finite difference time domain method for the design of birdcage RF head coils using multi-port excitations,” Mag. Reson. Im., 2000.
[3] T. Ibrahim et al., “Effect of RF coil excitation on field inhomogeneity at ultra high fields: a field optimized TEM resonator,” Mag. Reson. Im., 2000.
[4] G. Shajan et al., “An 8-channel transmit 32-channel receive 7T head coil for 1Tx and pTx scanner modes,” Proc. Int. Soc. Mag. Reson. Med., 2019.
[5] P. McElhinney et al., “Computational simulation of an 8Tx/32Rx open-face head coil for 7 tesla,” Proc. Brit. Chap. Int. Soc. Mag. Reson. Med., 2019.
[6] G. Eichfelder et al., “Local specific absorption rate control for parallel transmission by virtual observation points,” Mag. Reson. Med., 2011.
[7] J. Hoffmann et al., “Safety testing and operational procedures for self-developed radiofrequency coils,” NMR Biomed., 2015.
[8] P. Ehses et al., “MRI thermometry: fast mapping of RF-induced heating along conductive wires,” Mag. Reson. Med., 2008.
[9] S. Chung et al., "Rapid B1+ mapping using a preconditioning RF pulse with TurboFLASH readout," Mag. Reson. Med., 2010.
[10] A. Christ et al., “The virtual family—development of surface-based anatomical models of two adults and two children for dosimetric simulations,” Phys. Med. Biol., 2012.
[11] K. Setsompop et al., “Slice-selective RF pulses for in vivo B1 inhomogeneity mitigation at 7 tesla using parallel RF excitation with a 16-element coil, ”Mag. Reson. Med., 2008.
Figures