4036
Open-Sourced, Stereotax-Integrated, High-Fidelity and Adjustable 4-Channel Receive Array for Primate Brain Imaging at 3T
Marc Fischer1, Karthik Gopalan1, Ana Arias1, and Michael Lustig1
1EECS, UC Berkeley, Berkeley, CA, United States
1EECS, UC Berkeley, Berkeley, CA, United States
Synopsis
High quality MRI scans of nonhuman primates allow researchers to gain a deeper understanding of the brain. Most commercially available coils are designed for human imaging and are not compatible with common animal head restraint systems. We describe the design and testing of a 4-channel coil array that interfaces with a commercially available sterotax. Each channel is integrated with a modified Loc-line that enables the positioning of each coil independently in order to get the coils as close to the subject as possible. SNR maps reveal exceptional performance and good coil decoupling compared to three commercially available human coils.
Introduction
Imaging nonhuman primates (NHP) is of importance for neuroscience and preclinical work studying different brain functions and diseases. Many groups use MRI to get high-resolution anatomical scans of these subjects. MRI uses arrays of RF coils in order to detect a signal, but common commercially available receive coils are designed for humans. Primate imaging with these coils results in poor SNR and low image quality due to the size difference of the subjects. Some groups use a stereotax, a bulky head-restraint system, to hold the subject in place. This typically limits the coil selection to only the body coil. NHP head sizes vary significantly so a fixed array would always be suboptimal. To address these challenges, we designed and built a 4-channel receive array that interfaces with a commercially available stereotax. Our design allows the operator to independently place the coils in different locations and orientations in space. We rely on preamplifier decoupling to limit coil interactions during scanning. We show that, due to closer coil placement, our array significantly outperforms commercially available human coils when tested on a 100 mm diameter spherical phantom.Methods
We designed around the Kopf Model 1430M Stereotaxic Instrument, an NHP head-restraint system (Fig. 1). All parts were modelled in Autodesk Fusion 360 and printed with the Formlabs Form 2 3D printer in rigid, clear, and white resin (Fig. 2). The coils were mounted on the instrument’s guide rails with blocks that can be slid along the length of the stereotax. Each channel is placed at the end of a piece of Loc-Line, a modular hose system intended for positioning coolant lines during CNC machining. The coaxial cable runs through the Loc-Line and permits the user to place the coil in different orientations in free space. The enclosure of the coil features a tapered design that interfaces with the Loc-Line, allows for closer coil placement, and protects the Q-spoiling and matching circuitry. The 8 cm circular coil PCBs were designed in Eagle and manufactured by PCBway. Each channel used the standard 3T topology reported by Wiggins et al [1]. Three-quarter wavelength RG316 cables are used to connect the coils to a 4-channel low impedance preamp interface box (Stark Contrast). We created a floating trap balun design (Fig. 2c) that clamped around the Loc-Line. This design allowed us to place the trap as close to the coil as possible while retaining the maneuverability of the system. Two more traps were placed around each cable and attached with Kapton tape.Benchtop measurements were performed with an Agilent E5061B network analyzer with a geometrically decoupled double probe (design from the Wald group at MGH). Data were acquired on a Siemens 3T Trio scanner using a 10 cm diameter spherical phantom (GE Healthcare). Gradient echo scans (TE/TR/$$$\theta$$$ = 3.92ms/200ms/20$$$^{\circ}$$$, voxel = 1 x 1 x 10 mm$$$^3$$$, matrix = 128 x 128 [1]) were performed with the proposed coil array, a 12-channel volume coil, a 32-channel head coil, and the body coil. Noise was measured with the same scan parameters but with the transmit voltage adjusted to 0V. All SNR maps were calculated by dividing the root sum of squares reconstructed images by the standard deviations of the noise images [6]. Noise correlation coefficient matrices for two coil orientations were computed in Matlab with data acquired with the rf_noise service sequence.
Results and Discussion
Benchtop measurements revealed unloaded/loaded Q ratios between 8 and 12 indicating body noise dominance. Preamplifier decoupling limited the current in the loops by about 17dB for each channel. The q-spoiling circuitry provided a blocking of greater than 40 dB between the loaded and de-tuned states (Fig. 3). The mean off diagonal noise correlation coefficients were around 0.41 for both tested coil orientations (Fig. 4). Scanning revealed high SNR throughout the phantom (Fig. 5).The highlight of the work presented here is the shape-adaptable mechanical design. We have developed a way to place individual channels in any location in space. This design is compatible with NHPs of varying sizes and fits around the stereotaxic apparatus. SNR maps emphasize the importance of placing coils as close to the subject as possible. Our coil array exhibited higher noise correlation coefficients and more coupling compared to other coil arrays. Despite this, we expect the SNR gain from placing the coils closer to the subject outweighs the importance of improved decoupling. The channels can be corrected with post processing techniques such as noise prewhitening [3].
Conclusion
We built a 4-channel coil array to address the needs of an NHP imaging lab at our institution. Our coil design allows the operator to place each channel as close to the subject as possible without interfering with the head restraint system. Despite increased coupling, the coils exhibited significantly higher SNR than the commercially available coils designed for human imaging. While these coils were designed for NHP, similar designs could be used and extended for humans. While human applications may require higher channel counts, we do not anticipate that it will pose significant increases in complexity. In the spirit of reproducible research, we have made all of our CAD files open-sourced at: https://github.com/mikgroup/NHP-Coil.Acknowledgements
We would like to express special thanks to Ekin Karaşan, Victor Han, and Jon Tamir, who helped to finalize this abstract. We also thank Jim Tropp, who helped with the coil design. A big thank you goes to the entire group whose culture fosters an environment of collaboration and innovation. They made this research project a very rich and enjoyable learning journey. Last but not least, we would also like to thank the Wallis Lab for presenting us with this interesting challenge.References
- Wiggins, G. , Triantafyllou, C. , Potthast, A. , Reykowski, A. , Nittka, M. and Wald, L. (2006), 32‐channel 3 Tesla receive‐only phased‐array head coil with soccer‐ball element geometry. Magn. Reson. Med., 56: 216-223. doi:10.1002/mrm.20925
- Kellman, Peter, and Elliot R. McVeigh. "Image reconstruction in SNR units: a general method for SNR measurement." Magnetic resonance in medicine 54.6 (2005): 1439-1447.
- Roemer, P. B., Edelstein, W. A., Hayes, C. E., Souza, S. P. and Mueller, O. M. (1990), The NMR phased array. Magn Reson Med, 16: 192-225. doi:10.1002/mrm.1910160203
- Corea, J., Flynn, A., Lechêne, B. et al. Screen-printed flexible MRI receive coils. Nat Commun 7, 10839 (2016) doi:10.1038/ncomms10839
- Corea, J., “Screen Printed MRI Receive Coils”, Doctoral Thesis, Department of Electrical Engineering and Computer Science. 2016.
- Dietrich, O. , Raya, J. G., Reeder, S. B., Reiser, M. F. and Schoenberg, S. O. (2007), Measurement of signal‐to‐noise ratios in MR images: Influence of multichannel coils, parallel imaging, and reconstruction filters. J. Magn. Reson. Imaging, 26: 375-385. doi:10.1002/jmri.20969
- Keil, Boris. “Construction of Receive Arrays.” ISMRM 13 Weekend Educational Course.
Figures
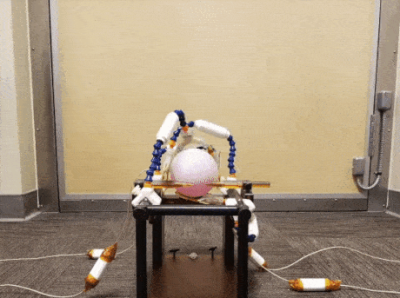
Animation showcasing the maneuverability of our coil design.The phantom is placed where the NHP’s head would be. The coils can surround the phantom fully, while there is space for the NHP’s body in the back of the stereotax.
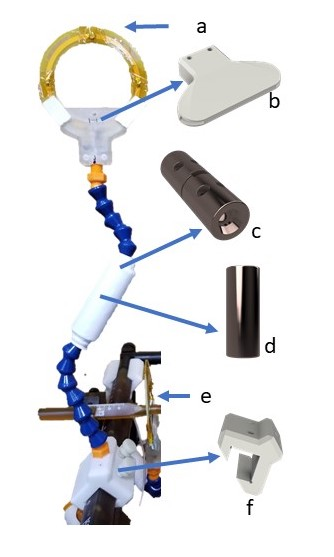
(a) Each coil employs the standard 3T topology. The loop design allows it to be placed over the ear bars (e). (b) A tapered enclosure holds the coil and protects the blocking circuit from degradation. (c-d) A Loc-line compatible 3D printed shielded floating trap. (f) A slidable and turnable attachment.
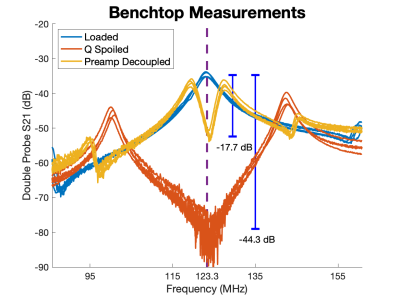
Benchtop tests were conducted with a double probe to measure the frequency response of the coils loaded on a conductive phantom. The detuned state was measured by applying 100 mA of current through the pin diode. Preamp decoupling was measured by plugging the coils into our interface box.
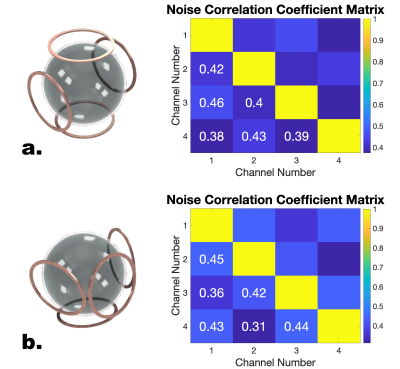
Noise correlation coefficient matrices for two coil orientations. The mean noise correlation coefficient was 0.41 for both setups. Even though this was higher than expected, we noticed significant SNR gains by placing the coils closer to the subject. The coupling could be mitigated with adaptive coil combination.
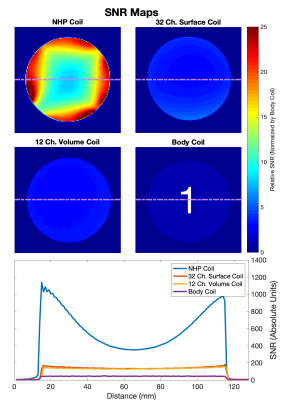
SNR maps generated from axial gradient echo scans. The four images are normalized by the mean SNR in the center of the body coil image. Our coil has significantly higher SNR compared to the 12 channel volume coil, the 32 channel surface coil, and the body coil available at our scanner suite. The SNR profile through the center of the slice reveals that our coil performs 2-5 times better than the other coils.