4012
Amplitude modulation of cardiovascular brain pulsations1Research Unit of Medical Imaging Physics and Technology (MIPT), University of Oulu, Oulu, Finland
Synopsis
Ultra-fast 10 Hz whole brain fMRI with magnetic resonance encephalography (MREG) enables separation of cardiovascular waves from brain pulsation without temporal aliasing. This enables analysis of spectral characteristics of respiration and cardiovascular pulsations by extending ALFF and fALFF methods to cardiovascular and respiration frequencies . Modulation of the cardiovascular pulsation has been detected in ECG and SpO2 and in this study show the modulation of the cardiovascular brain pulsation in 3D brain in healthy controls and how age and blood pressure affects the modulation.
Introduction
In this study we investigated the power distributions of each brain pulsation mechanism. The spatial distribution of the cardiovascular and respiratory were mapped using amplitude spectral map using established ALFF and fALFF techniques extended to cardiovascular and respiratory frequencies.1 Due to the fast scanning method, we were able to differentiate of cardiorespiratory brain pulsations known to exist in CSF pressure and flow. Also, we investigate how respiration return oscillations modulate cardiovascular pulses of the brain. This modulation has been shown to occur on three different forms: baseline, amplitude and frequency modulation, the last one known better as the heart rate variability [ref]. Mostly these modulations have been detected in electrocardiogram (ECG) and peripheral O2 saturation (SpO2) curves, but also in fast MRI techniques.3,4 Finally, we did linear regression analysis to show effects of blood pressure and age on the brain pulsations.Methods
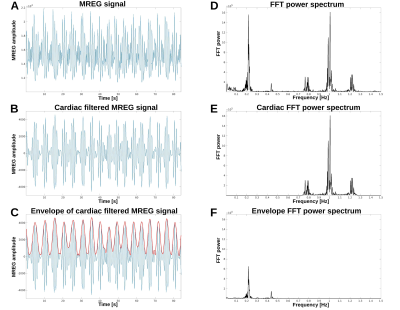
A total of 48/52 healthy subjects (age: 41.1 ± 17.2, 29 females) were scanned using 3T Skyra MREG sequence (TR=100 ms, TE=36 ms, flip angle=25°, 3D matrix=643, FOV=192 mm) for 5 to 10 min. First 6 s was discarded, and all data sets were set to 4.8 min (2880 volumes). Data was despiked using AFNI 3dDepsiked with –NEW option and processed with typical FSL pre-processing steps (Fig 1 a).5 During image preprocessing four subject were excluded because of defective images. The remaining 48 subjects were used in this study.Preprocessed MREG data frequency bands were inspected by using AFNI 3dPeriodogram to detect cardiac and respiratory peaks (Fig 1 d). Location of sidebands were calculated by adding/subtracting respiration peak frequency from cardiac peak frequency. LF was from 0.01 Hz to 1.0 Hz. Respiration and cardiac bands were set to 0.1 Hz band centered around the peaks. Locations of cardiac side peaks were calculated using heterodyne principle: multiplied sinusoidal waveforms maybe written as the sum and the difference of the multiplied frequencies.6 In our case frequencies are f(card) +/- f(respiration). Frequency bands of side peaks were also set to 0.1 Hz centered around peaks.
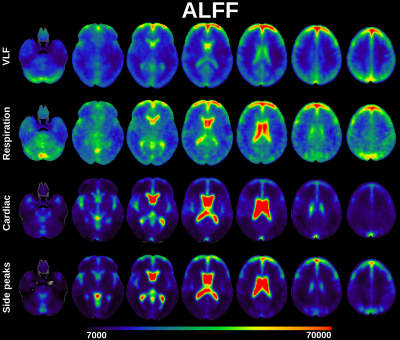
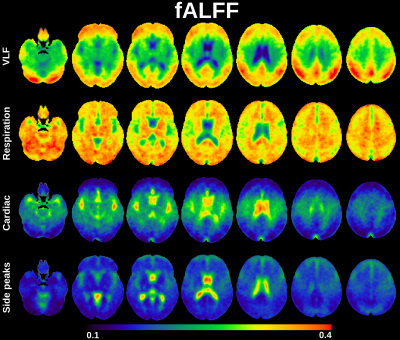
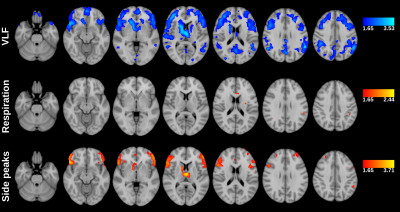
ALFF was computed from 3dperiodogram calculating square root and taking sum over frequency bands of interest: VLF, respiration, cardiac and side peaks (Fig 2). fALFF was calculated taking sum of frequency band of interest and dividing it by sum of all frequency bands of interest (Fig 3).
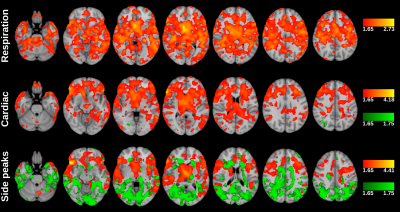
Regression analysis was performed for 43 subjects as 5 subjects didn’t have blood pressure information. Analysis were done for both ALFF (Fig 4) and fALFF (Fig 5). Voxel-wise comparisons were performed one-sample t-test using non-parametric permutation tests (10000 permutation) implemented vlisa_onesample in LISA.7
Results
Classical ALFF in very low frequencies (VLF < 0.1 Hz) occurs exactly in the previously described cortical areas dominantly in default mode and visual areas and frontal cortex.1The cardiac ALFF extends significantly in the periarterial areas along anterior, posterior and medial cerebral arteries and immediately adjacent perivascular spaces. The strong cardiac pulsation extends in the midline and lateral, 3rd and 4th CSF ventricles. Basal CSF spaces around brain stem, pons and basal cisternae are also strongly pulsating with cardiac frequency. Importantly cardiovascular pulsation also clearly present in posterior parts of sagittal sinus.
The respiratory ALFF pulsation is relatively strong over the whole brain tissue. Only periarterial structures and ventricles have relatively reduced respiratory power. Also, the cortical structures that have high VLF amplitude have relatively strong respiratory pulsations in addition to VLF. The respiratory power also extends to white matter quite uniformly.
The cardiac pulsation is modulated strongly by respiration in centrobasal CSF and periartial spaces. Modulation of cardiac brain pulsation by respiration occurs mostly in lateral and 3rd ventricle and periarterial spaces in areas where the cardiac pulsation frequency is also strong. In frontolateral areas the modulation also enters in ventromedial prefrontal parts of default mode network, frontal and sensorimotor cortices bilaterally, in posterior fossa cerebellar vermis, medulla and especially spinal cord.
Pulse pressure correlates more strongly with side peaks than with fundamental cardiac frequency. When comparing proportion of side peaks power to fundamental frequency with pulse pressure areas that show positive correlation are in arterial spaces.
Discussion
The critically sampled data allows aliasing free spectral analytics and the results indicate that the VLF distribution is not significantly affected by cardiorespiratory aliasing.8 The intracranial space and they induce CSF and brain tissue pulsations. The relative power of venous counter pulsations and CSF flow pulses into the brain tissue is to be determined. The strength of the modulation is governed by the elastic properties of the pulsating substance: the CSF pulses and modulations are stronger due to smaller resistance than in the brain tissue. Furthermore, the pulse pressure (I.e. systolic – diastolic BP) of the arteries governs the cardiovascular pulsation amplitude that then becomes modulated by the respiration.Conclusion
Respiratory modulate cardiac brain pulsations in CSF spaces, frontal brain cortex and in cerebellar/medulla areas. Physiologically the respiration controls venous return and CSF pulses, which then modulate arterial pulses in the brain vault. As the cardiac brain pulsation has been shown to be a main driver of the glymphatic brain clearance, the results add further proof that also respiration affects brain clearance.Acknowledgements
No acknowledgement found.References
1. Zou, Qi-Hong, et al. "An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF." Journal of neuroscience methods 172.1 (2008): 137-141.
2. Saykrs, B. McA. "Analysis of heart rate variability." Ergonomics 16.1 (1973): 17-32.
3. Charlton, Peter H., et al. "Extraction of respiratory signals from the electrocardiogram and photoplethysmogram: technical and physiological determinants." Physiological measurement 38.5 (2017): 669.
4. Abi-Abdallah, Dima, et al. "Cardiac and respiratory MRI gating using combined wavelet sub-band decomposition and adaptive filtering." Annals of biomedical engineering 35.5 (2007): 733-743.
5. Jenkinson, Mark, et al. "Fsl." Neuroimage 62.2 (2012): 782-790.
6. Graf, R. (1999), Modern dictionary of electronics, 7th Ed. USA: Newnes. p. 344. ISBN 0-7506-9866-7
7. Lohmann, Gabriele, et al. "LIPSIA—a new software system for the evaluation of functional magnetic resonance images of the human brain." Computerized medical imaging and graphics 25.6 (2001): 449-457.
8. Huotari, Niko, et al. "Sampling rate effects on resting state fMRI metrics." Frontiers in neuroscience 13 (2019): 279.
Figures