4002
The Neural Basis for Olfactory Deficits in Alzheimer’s Disease1Psychiatry, Penn State University, Hershey, PA, United States, 2Radiology, Penn State University, Hershey, PA, United States, 3Neurology, Penn State University, Hershey, PA, United States
Synopsis
Deficits in odor-memory and odor-identification are among the first clinical symptoms of Alzheimer’s Disease (AD) and mild cognitive impairment (MCI); however, the neural basis of this observation remains unclear. In this study, we combined independent Component Analysis and olfactory functional magnetic resonance imaging to investigate the relationships between the olfactory network (ON) and the medial temporal lobe (MTL). Our study's results suggest that the functional connectivity between the MTL and ON may be aberrant in MCI and AD patients.
INTRODUCTION
In Alzheimer’s disease (AD), olfactory deficits precede clinically detectable changes in cognition1. For example, deficits in odor-memory and odor-identification are among the first clinical symptoms of AD. Furthermore, olfactory deficits have been shown to be predictive of progressive memory loss and dementia, a characteristic of AD1. However, the neural basis of this observation remains unclear. In this study, we combined independent Component Analysis (ICA) and olfactory functional magnetic resonance imaging (fMRI) to investigate the relationships between the olfactory network (ON) and the medial temporal lobe (MTL)2. During olfactory fMRI, we hypothesized that the MTL activity and connectivity with the ON would be significantly different between cognitively normal (CN), amnestic mild cognitive impairment (MCI) and AD subjects.METHODS
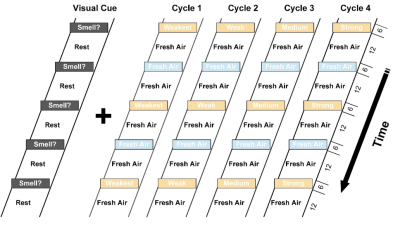
31 CN (mean age= 69.5 years, 15 F), 19 MCI (mean age= 72.8 years, 10 F) and 12 AD (mean age= 73.7 years, 8 F) subjects took part in the imaging study at 3T. We utilized an odor-visual association task within the MRI (Figure 1). Behaviorally, the participants’ smell and memory functions were evaluated using the University of Pennsylvania Smell Identification Test (UPSIT) and the Dementia Rating Scale (DRS), respectively3. Independent Component Analysis (ICA) was used to interrogate the interplay between the ON and the MTL during the odor-visual task in respective groups4.RESULTS
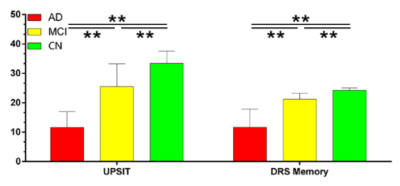
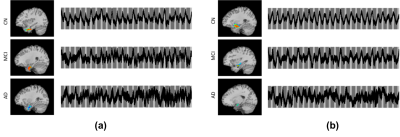
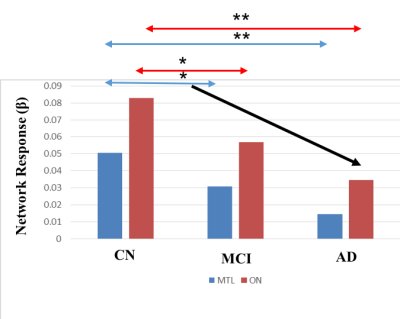
As shown in Figure 2, the UPSIT and DRS-memory scores in AD, MCI and CN groups showed group differences (p < 0.01). ICA maps in Figure 3ab provide support for our hypothesis, where patients demonstrated gradually decreasing fMRI activation in MCI and AD groups, which parallel their descending trend in UPSIT scores, as shown in Figure 2. MTL and ON shows periodic signal changes that follow the fMRI task timing structure. The amplitude of these fluctuations became smaller in magnitude and divergent (spread out) in MCI and AD subjects. These results suggest that the functional connectivity between the MTL and ON may be aberrant in MCI and AD patients. Furthermore, the fMRI activation is significantly different between CN and MCI in the two networks (Figure 4).Acknowledgements
The study was supported by the Leader Family Foundation, a grant from the U.S. NationalInstitute of Aging (R01-AG027771) and the Department of Radiology, Penn State College ofMedicine. This project also received partial funding from a grant with the PennsylvaniaDepartment of Health using Tobacco CURE Funds.References
1. Murphy, C. Olfactory and other sensory impairments in Alzheimer disease. Nature Reviews. Neurology. 2019; 15(1), 11–24. https ://doi.org/10.1038/s41582-018-0097-5.
2. Lech, R. K., & Suchan, B.The medial temporal lobe: memory and beyond. Behavioural Brain Research 2013; 254, 45-49.
3. Devanand, D. et al. Olfactory identification deficits and MCI in a multi‐ethnic elderly community sample. Neurobiology of Aging. 2010; 31(9), 1593–1600. https ://doi.org/10.1016/j.neuro biola ging.2008.09.008.
4. Karunanayaka et al. Networks involved in olfaction and their dynamics using independent component analysis and unified structural equation modeling. Human Brain Mapping. 2014; 35(5), 2055–2072. https ://doi.org/10.1002/hbm.22312
5. Jiaming Lu et al. Disruptions of the olfactory and default mode networks in Alzheimer's disease. Brain and Behavior. 2019;9(7):e01296. doi: 10.1002/brb3.1296. Epub 2019 Jun 4.
Figures