3999
Resting-state Functional MRI Investigation of Whiplash Associated Disorder1Northwestern University, Chicago, IL, United States, 2The University of Syndney, Sydney, Australia
Synopsis
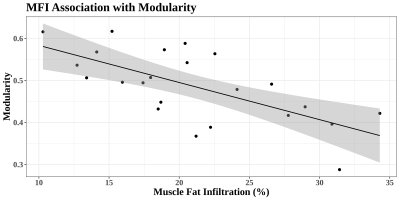
Many individuals who suffer an initial whiplash injury develop a chronic condition known as whiplash associated disorder (WAD). In this study, 23 participants with WAD underwent resting-state fMRI along with a fat-water scan of the cervical region to assess muscle fat infiltration (MFI), which has been demonstrated as a marker of disorder severity. Brain network modularity was calculated from the RS-fMRI data and associations between modularity and clinical measures were investigated. An association was discovered between modularity and MFI which appeared to be independent of demographic variables as well as scan motion.
Introduction
The term ‘whiplash’ refers to the transfer of force to the cervical spine via rapid acceleration-deceleration of the head. Whiplash injuries can result in a variety of persistent physiological and psychological sequelae collectively known as whiplash associated disorders (WAD).1 In addition to neck related symptoms, individuals with WAD may exhibit decreased performance on neuropsychological tests involving attention and working memory.2-4 Despite some evidence for cognitive and neurological symptoms, the presence of brain damage in this population is largely unknown. Conventional clinical imaging techniques have not revealed consistent markers of pathology in WAD.5-7 Some neuroimaging work has found changes in cerebral perfusion8, 9 and white matter tract integrity10, but investigations into neural correlates of WAD are in their infancy. In this study, resting-state fMRI was used to probe for associations between global brain network alterations and clinical measures within a group of participants with WAD. Included among more standard measures for assessment, an estimate of cervical muscle fat infiltration (MFI) was examined as an outcome, which has been previously validated as a sensitive measure of disorder severity.11-16Methods
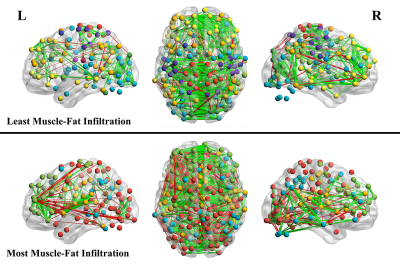
Twenty-three participants with WAD resulting from a motor vehicle collision at least 3 months prior were recruited and underwent MR imaging along with assessment of clinical scales. Resting state fMRI was acquired (TR=613ms, 2mm voxels, 800 volumes), along with T1w images (1mm) and 3D multiecho gradient-echo scans for quantification of MFI. The fMRI data was preprocessed using fmriprep v1.3.2.17 Briefly, data were normalized to standard MNI space, and a linear regression was used to remove 6 principal components of WM/CSF signals18, motion estimates, and low frequency cosine regressors. Volumes exceeding a specified motion threshold (≥0.5 framewise displacement) were removed along with one subsequent volume. The graph theoretical measure network modularity was calculated from the fMRI data. To derive modularity, network graphs were created for each participant consisting of seed-to-seed correlation matrices using the 5mm spheres defined by the Power 264 coordinates (reduced to 234 by removing those with tSNR < 2 SD below the mean).19 The matrices were converted to z-scores, and thresholds were used to minimize the number of node pairs considered to constitute edges to a percentage of the strongest connections. Connection densities used ranged from the strongest 2-10% of connections in increments of 1%, and an average across this range was taken for use in analysis19, 20 (though results across all individual thresholds showed similar effects). Newman’s spectral algorithm was used to divide the network into communities21, 22, and modularity (Q) was calculated from the weighted graph to quantify the extent to which the network was amenable to such subdivision. Higher values of Q represent more modular networks defined by a relatively high proportion of within-community connections to connections between communities. Muscle fat infiltration was calculated using the Dixon water-fat scan as previously described.23 Briefly, fat and water compartments of the bilateral multifidi and semispinalis muscles from C3-C7 were manually segmented. The mean voxel intensity within each compartment was extracted and MFI was then calculated as Fat/(Fat+Water)*100 to give a percentage of neck MFI for each subject. Potential associations between clinical measures and network modularity were investigated using multiple linear regression. Covariates in all models included age, body-mass-index, gender (coded 0/1), and mean framewise displacement (fMRI timeseries motion). Clinical metrics investigated included Neck Disability Index (NDI)24, Muscle fat infiltration (MFI), Depression Scale, Traumatic Injuries Distress Scale total (TIDS), Posttraumatic Diagnostic Scale hyperarousal symptom severity (PDS)25, numeric pain rating scale (0-10), and the Hospital Anxiety and Depression Scale Depression (HADS)26. Associations were considered significant if they passed Bonferroni correction (p≤0.007).Results
Among models including only the covariates, the only significant effect observed was of age on MFI, with greater age being associated with larger amounts of fat infiltration (t=3.17, p=0.005). In the full models, network modularity was found to be negatively associated with MFI (t=-4.02, partial R2=0.49, p=0.0009; Figures 1&2), and was not found to be associated with any other clinical metrics. No correlation was found between mean scan motion (FD) and modularity across participants (Pearson r=-0.21, p=0.34).Discussion & Conclusion
This study represents a first attempt at investigating whether RS-fMRI measures can characterize the clinical status of a heterogenous group of patients with WAD. While previous research in WAD has yet to accurately and consistently identify markers of structural pathology with conventional imaging, this investigation has found evidence of altered network structure in the brain using more advanced imaging techniques. The existence of altered network organization in WAD raises interesting considerations for future research and clinical practice. While most patients that suffer from a whiplash injury make a full recovery, many continue to exhibit symptoms for years following the event, and few quantitative tools are available for differentiating these groups in the acute stage.27, 28 There is still a need for metrics to identify individuals at risk of a slow or nonexistent recovery trajectory. The use of network modularity has the potential to capture a wide range of diverse network connectivity variations due to injury in a single global metric, and may prove to be a sensitive measure in conjunction with estimates of MFI.Acknowledgements
The authors wish to thank the Emergency Medicine Department at Northwestern Memorial Hospital, & Marie Wasielewski Chicago Illinois, USA for their assistance in the recruitment of acutely injured participants. We also wish to thank all participants for their involvement. The project described originated at Northwestern University, Feinberg School of Medicine, Department of Physical Therapy and Human Movement Sciences and supported by the National Institutes of Health (NIH) through Grant Number R01HD079076: Eunice Kennedy Shriver National Institute of Child Health & Human Development; National Center for Medical Rehabilitation Research (JME, TBP).References
1.Spitzer, W.O., et al., Scientific monograph of the Quebec Task Force on Whiplash-Associated Disorders: redefining "whiplash" and its management. Spine (Phila Pa 1976), 1995. 20(8 Suppl): p. 1S-73S.
2.Beeckmans, K., et al., Persistent cognitive deficits after whiplash injury: a comparative study with mild traumatic brain injury patients and healthy volunteers. Acta Neurol Belg, 2017. 117(2): p. 493-500.
3.Ettlin, T.M., et al., Cerebral symptoms after whiplash injury of the neck: a prospective clinical and neuropsychological study of whiplash injury. J Neurol Neurosurg Psychiatry, 1992. 55(10): p. 943-8.
4.Radanov, B.P., J. Dvorak, and L. Valach, Cognitive deficits in patients after soft tissue injury of the cervical spine. Spine (Phila Pa 1976), 1992. 17(2): p. 127-31.
5.Matsumoto, M., et al., Cross-sectional area of the posterior extensor muscles of the cervical spine in whiplash injury patients versus healthy volunteers--10 year follow-up MR study. Injury, 2012. 43(6): p. 912-6.
6.Matsumoto, M., et al., Modic changes of the cervical spine in patients with whiplash injury: a prospective 11-year follow-up study. Injury, 2013. 44(6): p. 819-24.
7.Ronnen, H.R., et al., Acute whiplash injury: is there a role for MR imaging?--a prospective study of 100 patients. Radiology, 1996. 201(1): p. 93-6.
8.Linnman, C., et al., Chronic whiplash symptoms are related to altered regional cerebral blood flow in the resting state. Eur J Pain, 2009. 13(1): p. 65-70.
9.Vallez Garcia, D., et al., Altered Regional Cerebral Blood Flow in Chronic Whiplash Associated Disorders. EBioMedicine, 2016.
10: p. 249-57.10.Jang, S.H. and Y.H. Kwon, A Review of Traumatic Axonal Injury following Whiplash Injury As Demonstrated by Diffusion Tensor Tractography. Front Neurol, 2018. 9: p. 57.
11.Abbott, R., et al., The geography of fatty infiltrates within the cervical multifidus and semispinalis cervicis in individuals with chronic whiplash-associated disorders. J Orthop Sports Phys Ther, 2015. 45(4): p. 281-8.
12.Abbott, R., et al., The qualitative grading of muscle fat infiltration in whiplash using fat and water magnetic resonance imaging. Spine J, 2018. 18(5): p. 717-725.
13.Elliott, J., et al., Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: a magnetic resonance imaging analysis. Spine (Phila Pa 1976), 2006. 31(22): p. E847-55.
14.Elliott, J., et al., Fatty infiltrate in the cervical extensor muscles is not a feature of chronic, insidious-onset neck pain. Clin Radiol, 2008. 63(6): p. 681-7.
15.Elliott, J.M., et al., Magnetic resonance imaging findings of fatty infiltrate in the cervical flexors in chronic whiplash. Spine (Phila Pa 1976), 2010. 35(9): p. 948-54.
16.Karlsson, A., et al., An Investigation of Fat Infiltration of the Multifidus Muscle in Patients With Severe Neck Symptoms Associated With Chronic Whiplash-Associated Disorder. J Orthop Sports Phys Ther, 2016. 46(10): p. 886-893.
17.Esteban, O., et al., fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods, 2019. 16(1): p. 111-116.
18.Behzadi, Y., et al., A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 2007. 37(1): p. 90-101.
19.Power, J.D., et al., Functional network organization of the human brain. Neuron, 2011. 72(4): p. 665-78.
20.Gallen, C.L., et al., Modular Brain Network Organization Predicts Response to Cognitive Training in Older Adults. PLoS One, 2016. 11(12): p. e0169015.
21.Newman, M.E., Modularity and community structure in networks. Proc Natl Acad Sci U S A, 2006. 103(23): p. 8577-82.
22.Reichardt, J. and S. Bornholdt, Statistical mechanics of community detection. Phys Rev E Stat Nonlin Soft Matter Phys, 2006. 74(1 Pt 2): p. 016110.
23.Elliott, J.M., et al., The Rapid and Progressive Degeneration of the Cervical Multifidus in Whiplash: An MRI Study of Fatty Infiltration. Spine (Phila Pa 1976), 2015. 40(12): p. E694-700.
24.Vernon, H. and S. Mior, The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther, 1991. 14(7): p. 409-15.
25.McCarthy, S., Post-Traumatic Stress Diagnostic Scale (PDS). Occupational Medicine, 2008. 58(5): p. 379-379.
26.Zigmond, A.S. and R.P. Snaith, The hospital anxiety and depression scale. Acta Psychiatr Scand, 1983. 67(6): p. 361-70.
27.Gargan, M.F. and G.C. Bannister, The rate of recovery following whiplash injury. Eur Spine J, 1994. 3(3): p. 162-4.28.Carroll, L.J., et al., Course and prognostic factors for neck pain in workers: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976), 2008. 33(4 Suppl): p. S93-100.
Figures