3998
Functional Anatomy of Human Brainstem using resting state fMRI1Department for High-field Magnetic Resonance, Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2Donders Institute for Brain, Cognition, and Behavior, Centre for Cognitive Neuroimaging, Nijmegen, Netherlands, 3Max Planck Institute for Biological Cybernetics, Tuebingen, Germany
Synopsis
The brainstem engages in various brain functions. However, we still lack a detailed understanding of its underlying functional organization. Therefore, in this study, we analyzed 222300 fMRI scans of 62 subjects acquired at the HCP-7 Tesla project. We applied the instantaneous parcellation analysis (ICP) method and determined a reliable, reproducible, and stable functional anatomy of the brainstem. The results reveal that stable multi-scale functional anatomy exists in the brainstem. The spatial rich functional anatomy enables neuroscientists to better characterize brainstem organization and understand its function and role in various brain disorders.
Introduction:
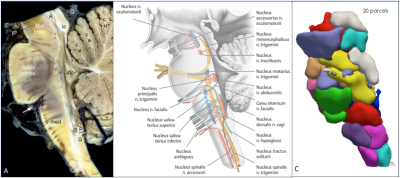
The brainstem serves as the connection between the cerebral hemispheres with the medulla and the cerebellum and is responsible for basic vital functions, such as breathing, heartbeat blood pressure, control of consciousness, and sleep1. The gray matter of the brainstem is organized in clumps and clusters to form the cranial nerve nuclei, the reticular formation, and pontine nuclei, while the white matter is composed of the axons of multiple pathways connecting the neurons of the cerebral cortex with the spinal cord, the cerebellum, and the cranial nerve nuclei (s. Fig. 1). The numerous brainstem nuclei mostly lie close together, resulting in a sophisticated anatomy2–4. They perform numerous functions and houses separate serotonergic, noradrenergic, dopaminergic, and cholinergic systems. We still know little about the detailed functional organization and diversity of the brainstem nuclei in humans. Therefore, we thought to examine whether high-resolution functional resting state MRI (r-fMRI) coupled with a refined functional parcellation method could produce a reliable, stable, and detailed functional anatomy of the brainstem nuclei.Material and Methods:
Data : Gradient-echo EPI was used to acquire the resting state fMRI (r-fMRI) data in HCP project5. TR: 1000 ms, TE: 22.2 ms, flip angle 45 deg, FOV: 208 x 208 mm (RO x PE), Matrix: 130 x 130 (RO x PE), slice thickness: 1.6 mm; 85 slices; 1.6 mm isotropic voxels, Multiband factor: 5, iPAT: 2, Partial Fourier (pF) sampling: 7/8, Echo spacing: 0.64 ms, Bandwidth: 1924 Hz/Px, 4 Runs, 900 frames per run, Duration/Run: 16:00 minutes5.Subjects: The data sample consisted of 62 young, healthy subjects (22-36 years old; genetically unrelated). Each subject performed four separate r-fMRI sessions, which were used for the analysis.
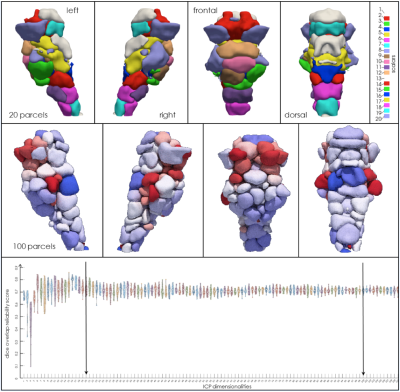
Data Analysis: The data were preprocessed using the HCP pipeline6. The instantaneous connectivity parcellation7 was performed. Subsequently, a split-half reproducibility analysis was performed to unravel the most robust and reproducible number of functionally similar parcels within the brainstem (s. Fig. 2).
Results:
The functional parcellation revealed the fine-grained organization of distinct functional units. The parcels varied in size, location, distribution, and reliability score (s. Fig. 2). However, the parcels appear in an organized modular distributed scheme. This variability within parcels seemingly reflects functional differences between local and global associations with other parts of the brain. A split-half reproducibility analysis revealed that the brainstem possesses not a unique dimension functional organization, but instead possess a preferably a multi-scale organization. A rigorous split-half reproducibility analysis indicates a first well-defined global peak at 20 parcels, which matches with the gross delineation of different substructures within the brainstem, i.e., the medulla, pons, and midbrain. As the dimensions of the parcels increase, we observed multiple reliable peaks up to 100 dimensions, which maintain a rough symmetry and characterize finer functional anatomy, seemingly delineating different functional properties for each nucleus at rest.Discussion:
The imaging of the brainstem in MR remains a yet challenging issue due to the low signal sensitivity of the brainstem, the increasing physiological artifacts, and partial volume effects8,9. Therefore only a few brainstem studies show the structural and functional organization of the brainstem10–12. In our study, we mapped the brainstem using state of the art method coupled with the high-resolution data obtained from the HCP project. However, numerous MR acquisition aspects (spatial resolution, distortion, temporal resolution, SNR and CNR, subject motion) remain and have to be solved in the future for better and ultra-high spatial-temporal resolution data, which is an essential requirement for the better understanding of the human brainstem13,14.Conclusion:
The group ICP analysis revealed a stable and robust functional parcellation of the brainstem into spatial symmetric and distributed parcels. The parcels showed variability in size, location, and symmetry. In the detailed comparison of the split-half reproducibility analysis, driven reliability scores revealed that the brainstem sub-structures exhibit spatially stable but multi-scale functional organizations at rest. The generated functional anatomy maps can have an impact on further research on degenerative and psychiatric brain disorders but await further a detailed functional assignment to the multitude of brainstem functions15.Acknowledgements
Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: D.C. Van Essen and K. Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. This work was supported by the DFG (grant number GZ: GR 833/11-1).References
1. Angeles Fernández-Gil, M., Palacios-Bote, R., Leo-Barahona, M. & Mora-Encinas, J. P. Anatomy of the brainstem: a gaze into the stem of life. Semin. Ultrasound. CT MR 31, 196–219 (2010).
2. Paxinos, G., Xu-Feng, H., Sengul, G. & Watson, C. Chapter 8 - Organization of Brainstem Nuclei. in The Human Nervous System (Third Edition) (eds. Mai, J. K. & Paxinos, G.) 260–327 (Academic Press, 2012).
3. Venkatraman, A., Edlow, B. L. & Immordino-Yang, M. H. The Brainstem in Emotion: A Review. Front. Neuroanat. 11, (2017).
4. Paxinos, G., Huang, X.-F., Sengul, G. & Watson, C. Organization of brainstem nuclei. (2012).
5. Van Essen, D. C. et al. The Human Connectome Project: a data acquisition perspective. NeuroImage 62, 2222–2231 (2012).
6. Glasser, M. F. et al. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage 80, 105–124 (2013).
7. van Oort, E. S. B. et al. Functional parcellation using time courses of instantaneous connectivity. NeuroImage 170, 31–40 (2018).
8. Forstmann, B. U., de Hollander, G., van Maanen, L., Alkemade, A. & Keuken, M. C. Towards a mechanistic understanding of the human subcortex. Nat. Rev. Neurosci. 18, 57–65 (2017).
9. Matt, E. et al. Improving sensitivity, specificity, and reproducibility of individual brainstem activation. Brain Struct. Funct. 224, 2823–2838 (2019).
10. Beissner, F., Schumann, A., Brunn, F., Eisenträger, D. & Bär, K.-J. Advances in functional magnetic resonance imaging of the human brainstem. NeuroImage 86, 91–98 (2014).
11. Deistung, A. et al. High-Resolution MR Imaging of the Human Brainstem In vivo at 7 Tesla. Front. Hum. Neurosci. 7, (2013).
12. Tang, Y., Sun, W., Toga, A. W., Ringman, J. M. & Shi, Y. A probabilistic atlas of human brainstem pathways based on connectome imaging data. NeuroImage 169, 227–239 (2018).
13. Beissner, F. Functional MRI of the Brainstem: Common Problems and their Solutions. Clin. Neuroradiol. 25 Suppl 2, 251–257 (2015).
14. Sclocco, R., Beissner, F., Bianciardi, M., Polimeni, J. R. & Napadow, V. Challenges and opportunities for brainstem neuroimaging with ultrahigh field MRI. NeuroImage 168, 412–426 (2018).
15. Hurley, R. A., Flashman, L. A., Chow, T. W. & Taber, K. H. The Brainstem: Anatomy, Assessment, and Clinical Syndromes. J. Neuropsychiatry Clin. Neurosci. 22, iv–7 (2010).
Figures