3996
Resting state fMRI signal complexity metrics indicate cerebellar cholinergic system damage in Gulf War Illness1Emory University, Atlanta, GA, United States, 2University of Houston Clear-Lake, Houston, TX, United States, 3UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
Around 200,000 veterans of the 1991 Gulf War (GW) suffer from GW illness (GWI), which is characterized by deficits in cognitive, emotion, perception and nociception domains. Previous studies have associated GWI with exposure to neurotoxic chemicals which impair the cholinergic system. Recently, an fMRI time-series signal complexity metric, multi-scale entropy (MSE) has been proposed as a potential biomarker of abnormal neural activity in brain disorders. In this study, we examined 23 GWI patients and 30 age-matched controls with resting state fMRI. GWI veterans exhibited abnormally increased MSE all across cerebellum, implicating cholinergic damage of cerebellum as a mechanism underlying GWI.
INTRODUCTION
Around 200,000 veterans (up to 32% of those deployed) of the 1991 Gulf War (GW) suffer from GW illness (GWI). GWI is a poorly understood chronic medical condition, characterized by multiple symptoms indicative of brain function deficits in cognitive, affective, perception and nociception domains 1-6. Epidemiologic and animal studies have associated GWI with exposure to neurotoxic chemicals such as nerve agents, organophosphate pesticides and pyridostigmine bromide, all of which are cholinergic stimulants that inhibit acetylcholinesterase 1,7,8. This increases acetylcholine concentration in the synaptic cleft; resulting in hyper-excitation of cholinergic synapses, which can cause persistent brain changes up to and including neuronal death 1,7-9. A number of brain regions including the cerebrum, striatum and cerebellum are richly innervated by cholinergic projections 10-12. A gap in the current state of knowledge is how cholinergic system impairment results in brain function deficits observed in GWI, since the effects of exposure to cholinergic stimulants can be heterogeneous across the brain 7,9. Recently, an fMRI time-series signal complexity metric, multi-scale entropy (MSE) has been proposed as a potential biomarker of neural activity and excitation/inhibition balance 13-15. In this study, we employed resting state fMRI (rsfMRI) to explore impairments in brain function networks in GWI with MSE.METHODS
23 GWI veterans (mean age 49.4 yrs.) and thirty healthy veteran controls (VC) (mean age 49.8 yrs.), were scanned in a Siemens 3T MRI scanner using a 12-channel Rx head coil. Written informed consent was obtained from all participants in the protocol approved by the local Institutional Review Board. RsMRI data were acquired with a 10-min whole-brain gradient echo EPI (TR/TE/FA = 2000/24ms/90°, resolution = 3mm x 3mm x 3.5mm). RsfMRI preprocessing steps included attenuation of signal related to subject-motion and physiological responses, using the AROMA technique 16, and spatial smoothing with FWHM = 6mm isotropic Gaussian kernel. Voxelwise sample entropy (SE) maps were evaluated at 10 temporal scales using the 3dMSE program in AFNI 17. The window-length (m) for MSE calculations was set to 2. In order to find the optimal distance threshold (r), MSE was evaluated at m=2, and r = 0.1 x SD to 1.5 x SD, in steps of 0.1 x SD (where SD was the standard deviation of the rsfMRI time-series) for all the VC rsfMRI datasets. Voxel MSEs were averaged across all grey matter voxels of all VCs. The plot of AUC of the resultant average MSE as a function of r, exhibited a maximum at r = 0.7 x SD, which was set as the optimal distance threshold for MSE calculations for all subjects. Between-group differences in complexity were obtained with separate 2-sample t-tests for SE at each scale. The resultant t-statistic maps were clustered and the inferences were corrected for multiple comparisons (mcc) through Monte-Carlo simulations explicitly accounting for the spatial correlation of second-level analysis residuals 18.RESULTS & DISCUSSION
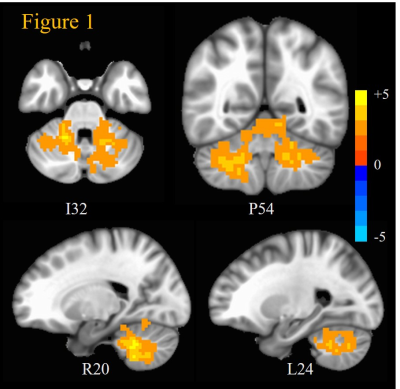
GWI veterans exhibited (Figure 1) significantly (mcc p < 0.05) increased SE at Scale 1 compared to VC across widespread regions in the cerebellum. MSE differences between the two groups in cerebrum and other sub-cortical regions did not achieve significance (at mcc p < 0.05). Thus, cerebellum seems to exhibit the most impairment in terms of hyper-excitation of cholinergic synapses in GWI. Cerebellum works in concert with basal ganglia, thalamus and cortex in the execution of almost all brain functions 19,20. Cerebellum projects to motor, somatosensory and auditory cortices 19-22, and cholinergic impairments indicated by these results may be the source of perceptual, perceptuomotor, and vestibular deficits, as well as confusion ataxia seen in GWI 2,8. Cerebellum is also known to take part in language 23 and other cognitive functions 23-26, which is consistent with deficits in these functions seen in GWI. Cholinergic impairment of cerebellum can also give rise to chronic pain symptoms 27, which are prevalent in GWI 4,8,28,29. These results are also consistent with structural and functional abnormalities in cerebellum observed by other groups 28,29.CONCLUSION
The results of this study implicate hyper-excitation of cholinergic synapses in cerebellum as a putative mechanism for GWI. Future work will extend this analysis to a larger GWI patient cohort, and also examine relationship between MSE and measures of neurotoxic exposure, as well as the neuroprotective PON1 gene expression in GWI.Acknowledgements
This work was supported by the Office of Assistant Secretary of Defense for Health Affairs, through the Gulf War Illness Research Program under Award No.W81XWH-16-1-0744. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.References
1. Binns JH, Barlow C, Bloom FE, Clauw DJ, Golomb BA, Graves JC. Report of Research Advisory Committee on Gulf War Veterans’ Illnesses. In: Affairs DoV, ed. Boston, MA: U.S. Government Printing Office; 2014.
2. Hom J, Haley RW, Kurt TL. Neuropsychological correlates of Gulf War syndrome. Arch Clin Neuropsychol 1997;12:531-44.
3. Calley CS, Kraut MA, Spence JS, Briggs RW, Haley RW, Hart J, Jr. The neuroanatomic correlates of semantic memory deficits in patients with Gulf War illnesses: a pilot study. Brain Imaging Behav 2010;4:248-55.
4. Gopinath K, Gandhi P, Goyal A, et al. FMRI reveals abnormal central processing of sensory and pain stimuli in ill Gulf War veterans. Neurotoxicology 2012;33:261-71.
5. Moffett K, Crosson B, Spence JS, et al. Word-finding impairment in veterans of the 1991 Persian Gulf War. Brain Cogn 2015;98:65-73.
6. Toomey R, Alpern R, Vasterling JJ, et al. Neuropsychological functioning of U.S. Gulf War veterans 10 years after the war. J Int Neuropsychol Soc 2009;15:717-29.
7. Bhardwaj S, Musalgaonkar N, Waghmare C, Bhattacharya BK. Single dose exposure of sarin and physostigmine differentially regulates expression of choline acetyltransferase and vesicular acetylcholine transporter in rat brain. Chem Biol Interact 2012;198:57-64.
8. Haley RW, Kurt TL, Hom J. Is there a Gulf War Syndrome? Searching for syndromes by factor analysis of symptoms. JAMA 1997;277:215-22.
9. Golomb BA. Acetylcholinesterase inhibitors and Gulf War illnesses. Proc Natl Acad Sci U S A 2008;105:4295-300.
10. Mesulam MM, Mash D, Hersh L, Bothwell M, Geula C. Cholinergic innervation of the human striatum, globus pallidus, subthalamic nucleus, substantia nigra, and red nucleus. J Comp Neurol 1992;323:252-68.
11. Stephenson AR, Edler MK, Erwin JM, et al. Cholinergic innervation of the basal ganglia in humans and other anthropoid primates. J Comp Neurol 2017;525:319-32.
12. Zhang C, Zhou P, Yuan T. The cholinergic system in the cerebellum: from structure to function. Rev Neurosci 2016;27:769-76.
13. Wang DJJ, Jann K, Fan C, et al. Neurophysiological Basis of Multi-Scale Entropy of Brain Complexity and Its Relationship With Functional Connectivity. Front Neurosci 2018;12:352.
14. Smith RX, Yan L, Wang DJ. Multiple time scale complexity analysis of resting state FMRI. Brain Imaging Behav 2014;8:284-91.
15. McDonough IM, Nashiro K. Network complexity as a measure of information processing across resting-state networks: evidence from the Human Connectome Project. Front Hum Neurosci 2014;8:409.
16. Pruim RH, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 2015;112:267-77.
17. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162-73.
18. Gopinath K, Krishnamurthy V, Sathian K. Accounting for Non-Gaussian Sources of Spatial Correlation in Parametric Functional Magnetic Resonance Imaging Paradigms I: Revisiting Cluster-Based Inferences. Brain Connect 2018;8:1-9.
19. Baumann O, Borra RJ, Bower JM, et al. Consensus paper: the role of the cerebellum in perceptual processes. Cerebellum 2015;14:197-220. 20. Bostan AC, Strick PL. The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci 2018;19:338-50.
21. Parsons LM, Petacchi A, Schmahmann JD, Bower JM. Pitch discrimination in cerebellar patients: evidence for a sensory deficit. Brain Res 2009;1303:84-96.
22. McLachlan NM, Wilson SJ. The Contribution of Brainstem and Cerebellar Pathways to Auditory Recognition. Front Psychol 2017;8:265.
23. Marien P, Ackermann H, Adamaszek M, et al. Consensus paper: Language and the cerebellum: an ongoing enigma. Cerebellum 2014;13:386-410.
24. E KH, Chen SH, Ho MH, Desmond JE. A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum Brain Mapp 2014;35:593-615.
25. Desmond JE, Fiez JA. Neuroimaging studies of the cerebellum: language, learning and memory. Trends Cogn Sci 1998;2:355-62.
26. Koziol LF, Budding D, Andreasen N, et al. Consensus paper: the cerebellum's role in movement and cognition. Cerebellum 2014;13:151-77.
27. Moulton EA, Schmahmann JD, Becerra L, Borsook D. The cerebellum and pain: passive integrator or active participator? Brain research reviews 2010;65:14-27.
28. Christova P, James LM, Engdahl BE, Lewis SM, Carpenter AF, Georgopoulos AP. Subcortical brain atrophy in Gulf War Illness. Exp Brain Res 2017.
29. Rayhan RU, Stevens BW, Raksit MP, et al. Exercise challenge in Gulf War Illness reveals two subgroups with altered brain structure and function. PLoS One 2013;8:e63903.