3993
Functional connectivity during spontaneous migraine attacks compared to pain-free periods: a resting-state fMRI study1ISR-Lisboa/LARSyS and Department of Bioengineering, Instituto Superior Técnico, Universidade de Lisboa, Lisboa, Portugal, 2Hospital da Luz, Lisboa, Portugal, 3Department of Clinical Neurosciences, Faculdade de Medicina, Universidade de Lisboa, Lisboa, Portugal
Synopsis
Migraine is a severe neurological brain condition of cyclical nature, with intermittent attacks alternating with attack-free periods. In this work, we studied a group of patients with episodic migraine without aura during a spontaneous migraine attack and during a pain-free period, using BOLD resting-state fMRI. Results showed decreases in functional connectivity (FC) within a sensorimotor-insular cortex network and a left frontoparietal/executive control network. These FC changes were correlated with the attack duration and the pain intensity of the ongoing attack, respectively.
Introduction
Migraine is a severe neurological brain condition of cyclical nature, with intermittent attacks alternating with attack-free periods. Attacks are characterized by a moderate to severe head pain, but migraineurs may also experience a variety of non-painful symptoms, probably associated with multiple cortical and subcortical brain regions. A number of resting-state fMRI (rs-fMRI) studies have reported changes in functional connectivity (FC) in several resting-state networks (RSNs) of migraineurs, often correlated with markers of disease burden 1. However, only two studies have analysed FC during spontaneous attacks and directly compared it to attack-free periods in the same patients 2,3. Here, we adopt a prospective longitudinal design and study a group of patients with episodic migraine without aura using rs-fMRI during a spontaneous attack (ictal) and a pain-free (interictal) period.Methods
Eleven female patients with episodic migraine without aura were recruited and blood oxygen level dependent contrast (BOLD) rs-fMRI data were collected from each patient in two sessions: during an ictal and an interictal phases. fMRI data were acquired on 3T Siemens Verio MRI system with a 12-channel RF coil using 2D-GE-EPI (TE/TR=30/2250ms, 3.5x3.5x6.25mm3 voxel size, ~7min). Several patients’ clinical features were collected for further correlation with imaging data, both characterizing usual migraine attacks or the ongoing attack during the ictal session.fMRI data were analysed using FSL. Preprocessing steps included: motion correction, distortion correction, spatial smoothing (FWHM=5mm), high-pass temporal filtering (cutoff-frequency=0.01Hz), nuisance regression of motion outliers and ICA denoising, as well as co-registration to MNI152 space. Probabilistic group-level ICA was performed across patients and sessions using FSL’s MELODIC, and 21 independent components (ICs) were classified as being of interest based on visual inspection, spacio-temporal characteristics and similarity to previously reported RSNs 4. Dual-regression analysis was then performed to generate session- and subject-specific FC maps for each of the 21 ICs, and the average FC within each IC was computed for each subject and session. Comparison of the IC's mean FC between the two sessions was performed using a two-sided Wilcoxon signed rank test (p-value<0.05, FDR-corrected). Finally, a post-hoc analysis was performed regarding the ICs for which the mean FC was significantly different between sessions, in order to test for relations between within-network FC and several clinical features (Spearman rank correlation, p-value<0.05 uncorrected for multiple comparisons).
Results
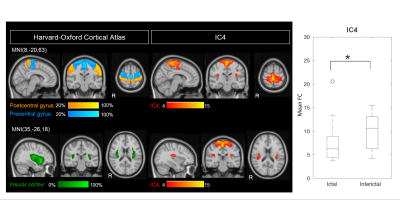
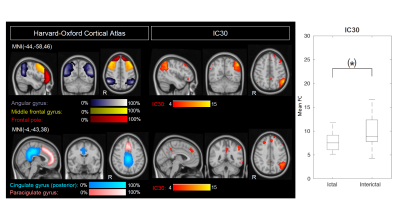
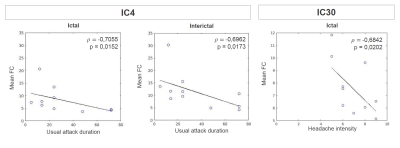
The high dimensionality of the group-ICA, in comparison to previously reported RSNs, allowed for detection of fine-grained RSNs, with increased potential on providing detailed insight into disease-related FC change. A significant difference between sessions was found for the mean FC of IC4 (p=0.04, FDR-corrected), which corresponds to the sensorimotor and bilateral posterior insular cortices (referred to here as sensorimotor-insular network) (Fig.1). A trend was also found for IC30 (p=0.02, uncorrected), which corresponds to the left frontoparietal/executive control network (Fig.2). Both networks exhibited decreased FC during the attack relative to the pain-free period. Post-hoc correlation analyses revealed that the mean FC in IC4 was negatively correlated with usual attack duration during both the ictal and interictal sessions (Fig.3-Left). Moreover, the mean FC in IC30 during the ictal phase was negatively correlated with the headache intensity of the ongoing attack (Fig.3-Right).Discussion
Regarding the observed changes in the FC of the sensorimotor-insular network (IC4), these might be related to the role of the sensorimotor network in pain processing, while the insula is associated to a wide variety of functions, including a potential involvement in sensorimotor integration and pain perception. Therefore, a differential interaction between the sensorimotor cortex and the posterior insula during migraine attacks in relation to attack-free periods could be responsible for abnormalities in pain perception, resulting in enhanced pain experience. To our knowledge, only one study has compared the sensorimotor network between induced-migraine attacks and interictal periods 5, and ours is therefore the first to report on spontaneous attacks.Regarding the changes in the FC of the left frontoparietal/executive control network (IC30), these might be explained by the fact that migraineurs have shown decreased cognitive performance during attacks and it has been hypothesized that such dysfunction could result from pain-cognition interactions 6. The observed tendency for the network's FC to decrease during migraine attacks could possibly result from pain-cognition interactions, with nociceptive inputs during head pain hindering the normal functioning of executive networks. Such hypothesis is further supported by the observed negative correlation between this networks' FC and headache intensity of the ongoing attack. To our knowledge, this is the first study to report changes in this network in ictal versus interictal periods.
Conclusion
We found decreases in FC within a sensorimotor-insular cortex network and within a left frontoparietal/executive control network, during spontaneous migraine attacks compared with pain-free periods, in a group of patients with episodic migraine without aura. The FC changes were correlated with the usual attack duration and the pain intensity of the ongoing attack, respectively. Overall, these results suggest that these networks may be involved in migraine attack mechanisms, and contribute new evidence to the limited number of studies investigating FC of migraineurs during attacks.Acknowledgements
We acknowledge funding by the Sociedade Portuguesa de Cefaleias—Tecnifar Headache Grant, and by the Portuguese Science Foundation (FCT) through Grants LISBOA-01-0145-FEDER-029675 and UID/EEA/50009/2019.References
1. C. D. Chong, T. J. Schwedt, and A. Hougaard. Brain functional connectivity in headache disorders: a narrative review of mri investigations. Journal of Cerebral Blood Flow & Metabolism, 39(4):650–669, 2019.
2. A. E. Edes, L. R. Kozak, M. Magyar, T. Zsombok, G. Kokonyei, G. Bagdy, and G. Juhasz. Spontaneous migraine attack causes alterations in default mode network connectivity: a resting-state fmri case report. BMC research notes, 10(1):165, 2017.
3. F. M. Amin, A. Hougaard, S. Magon, T. Sprenger, F. Wolfram, E. Rostrup, and M. Ashina. Altered thalamic connectivity during spontaneous attacks of migraine without aura: a resting-state fmri study. Cephalalgia, 38(7):1237–1244, 2018.
4. S. M. Smith, P. T. Fox, K. L. Miller, D. C. Glahn, P. M. Fox, C. E. Mackay, N. Filippini, K. E. Watkins, R. Toro, A. R. Laird, et al. Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences, 106(31):13040–13045, 2009.
5. F. M. Amin, A. Hougaard, S. Magon, M. S. Asghar, N. N. Ahmad, E. Rostrup, T. Sprenger, and M. Ashina. Change in brain network connectivity during pacap38-induced migraine attacks: a resting-state functional mri study. Neurology, 86(2):180–187, 2016.
6. R. Gil-Gouveia, A. G. Oliveira, and I. P. Martins. Cognitive dysfunction during migraine attacks: a study on migraine without aura. Cephalalgia, 35(8):662–674, 2015.
Figures