3989
Behavioral performance prediction in aging with advanced resting-state imaging acquisitions1Functional MRI Laboratory, University of Michigan, Ann Arbor, MI, United States, 2Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States, 3Neurology, University of Michigan, Ann Arbor, MI, United States, 4Psychiatry, University of Michigan, Ann Arbor, MI, United States, 5Mental Health Service, VA Ann Arbor Healthcare System, Ann Arbor, MI, United States
Synopsis
Multi-band resting state data using two different protocols was collected in older subjects. Connectome-based predictive modeling yielded significant fits for measures of learning, memory, and language; with higher spatiotemporal sampling being more sensitive.
INTRODUCTION
Resting-state connectivity in fMRI has been used to characterize changes in both healthy controls and pathological states. Low-frequency functional MRI connectivity is thus important as a potential indicator of activity in the brain, and can be used to develop predictive biomarkers for individual-based precision medicine [1].Connectome-based Predictive Modeling (CPM) has shown promise in relating single and multiple modality imaging-derived measures to clinical/behavioral observations in patient populations [2-4]. Recent work has demonstrated the utility of this approach in the MCI/AD population, finding relationships in behavioral measures of cognitive function and memory [5,6].
In this work, we extend this work using CPM for behavioral prediction of learning, memory and language, using two different resting-state acquisitions for each subject, in a cohort of cognitively intact older adults (CI), individuals with amnestic MCI (aMCI), and those with dementia of the Alzheimer’s type (DAT).
METHODS
Subjects: 70 older adults (age=69.5 (±7.4) years old) were enrolled at the Michigan Alzheimer’s Disease Consortium Center, CI (n=28), aMCI (n=29), DAT (n=19), who are part of the longitudinal University of Michigan Memory in Aging Program (UMMAP).Behavioral measures: Composite measures were formed for learning (average of Craft Story and Hopkins Verbal Learning Test – HVLT – immediate recall),memory (averaged scores of delayed recall in Craft story, Hopkins Verbal Learning Test, Benson Complex Figure), and language (average of phonemic and semantic fluency and confrontation naming).
MRI Acquisition: Resting-state fMRI scans were collected using a 3T GE MR750 scanner, using two alternative multiband acquisitions:
1) MB=3, TR=0.9s, resolution 3.24mm x3.24mm x3mm, 45 slices. This acquisition has larger voxel size with higher SNR similar to the ADNI 3 Basic protocol [7], but has the advantages of increased temporal sampling.
2) MB=6, TR=0.8s, resolution 2.4mm isotropic, 60 slices. This acquisition matches the ABCD consortium protocol [8], with increased spatial and temporal resolution.
Whole-brain T1 SPGR sequences were acquired for anatomical localization and normalization.
Preprocessing: Physiological noise removal was done using RETROICOR [9], slice-time correction was done using SPM8, and motion correction was done using FSL. Segmentation and normalization was done using VBM8. Spatial smoothing was implemented using SPM8. Connectivity matrices were then generated using the CONN toolbox [10], including band-pass filtering (0.01-0.10 Hz) to limit the analysis to resting-state frequencies of interest [11], and parcellation using the Power atlas [12] as well eight additional ROIs in L/R amygdala and hippocampus region. The resultant connectivity matrices and the behavioral data were orthogonalized to subject age using the Gram-Schmidt algorithm.
Data analysis: CPM analysis was applied following the protocol prescribed in Shen 2017 [4]. A leave-one-out framework was used to predict the behavioral score for each subject, using the rest of the data to generate the linear model at each iteration. For each set of training data, the resting-state connectivity matrix values were correlated across subjects with the behavioral score, then thresholded at 0.01 significance (feature reduction). The original raw connectivity values for those identified features were then summed to give an overall brain score, which was linearly fit to the behavioral score (done separately for positive and negative correlations with the behavioral score). This fit model was then used to generate a predicted score for the left-out subject. This process was independently done for each behavioral measure and acquisition method.
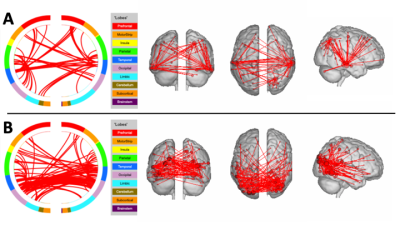
Goodness of fit was measured as the correlation between the predicted and actual behavorial scores. Permutation was used to assess significance, using 1000 iterations of this process with randomized labels, to generate a null distribution. Significant model connections were visualized using the Yale Bioimage Suite (bisweb.yale.edu) to generate brain lobe circle plots and glass brain node images.
RESULTS
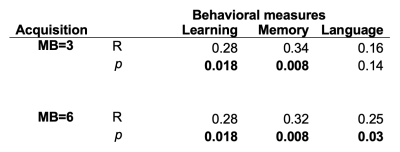
Table I displays the correlaton between the actual and predicted values for each behavioral measure, across acquisitions. Significant results for the learning measure were found for both the MB=3 and MB=6, showing reproducibility across acquisitions. The memory measure showed a significant result for MB=3, replicating earlier results [6], but here extended also to MB=6, again showing reproducibility across acquisition types. Additionally, the MB=6 acquisition found a significant relationship for the language measure. All significant results were found using positive edges, meaning increased connectivity resulted in increased scores (higher functioning). No significant results were found using the negative edges.Examples of the significant connections for the CPM models are shown in Figure 1, which displays the connections coming from the top five model nodes in terms of degree of connection.The CPM model for learning (Fig 1A) has highly connected nodes in the temporal lobes, known to be important for learning and memory processes. The CPM model for language (Fig. 1B) has highly connected nodes in visual processing and language areas, including occipital lobe, middle temporal gyrus, and fusiform gyrus.
DISCUSSION
This study establishes that connectome predictive modeling using resting state scans in older subjects can be consistent across acquisition parameters; but scans with higher spatiotemporal sampling may be optimal for prediction of functioning across multiple cognitive domains. It has also been demonstrated that behavioral metrics of learning and language can be predicted in this population using CPM and resting-state fMRI.Future work will examine the relative connectivity differences between scan types and integrate complementary disease-related biomarkers.
Acknowledgements
This work was supported by funding from the National Institute on Aging: Michigan Alzheimer’s Disease Research Center (5P30 AG053760);and from NIH R01 AG058724 (BMH).References
1) Craddock RC, Holtzheimer III PE, Hu XP, Mayberg HS. Disease state prediction from resting state functional connectivity. Magnetic Resonance in Medicine. 2009, 62:1619.
2) Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, Papademetris X, Constable RT. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nature neuroscience. 2015, 18:1664.
3) Rosenberg, M.D. et al. A neuromarker of sustained attention from whole-brain functional connectivity. Nat Neurosci. 2016, 19:165.
4) Shen X, Finn ES, Scheinost D, Rosenberg MD, Chun MM, Papademetris X, Constable RT. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. nature protocols. 2017, 12:506.
5) Lin Q, Rosenberg MD, Yoo K, Hsu TW, O'Connell TP, Chun MM. Resting-State Functional Connectivity Predicts Cognitive Impairment Related to Alzheimer's Disease. Frontiers in aging neuroscience. 2018, 10:94.
6) Peltier S, Ma S, Giordani B, Paulson H, Snyder R, Hampstead B. Predicting memory impairment using resting-state brain connectomes in older adults, in Proceedings of ISMRM 2019, 881.
7) Gunter JL, Borowski BJ, Thostenson K, Arani A, Reid RI, Cash DM, Thomas DL, Zhang H, DeCarli CS, Fox NC, Thompson PM. ADNI-3 MRI ACQUISITIONS. Alzheimer's & Dementia: The Journal of the Alzheimer's Association. 2017 13:P1368.
8) Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, Soules ME, Teslovich T, Dellarco DV, Garavan H, Orr CA. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Developmental cognitive neuroscience. 2018 32:43.
9) Glover GH, Li TQ, Ress D. Image‐based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magnetic Resonance in Medicine. 2000, 44:162.
10) Jelsone-Swain LM, Fling BW, Seidler RD, Hovatter R, Gruis K, Welsh RC. Reduced interhemispheric functional connectivity in the motor cortex during rest in limb-onset amyotrophic lateral sclerosis. Frontiers in systems neuroscience. 2010, 4:158.
11) Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Mapping functionally related regions of brain with functional connectivity MR imaging. Am J Neuroradiol. 2000, 21:1636.
12) Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron. 2011, 72:665.
Figures