3988
Seed-based resting state fMRI data analysis pipeline by using unsupervised machine learning1Diagnostic Radiology, Cleveland Clinic, Cleveland, OH, United States
Synopsis
We have developed an automatic pipeline to generate seed clusters and corresponding maps for rs-fMRI data analysis by using unsupervised machine learning method. It only needs manual participation in the end to review the candidate seed cluster locations and connectivity maps to make decision. In contrast to common anatomical seed scheme which usually consists of a small neighborhood surrounding a single voxel, seeds in our pipeline were determined functionally within large pre-defined ROI which can be derived by using automatic brain segmentation tools like FreeSurfer. The pipeline was tested successfully on rs-fMRI studies with accompanied task-based fMRI involving motor cortex.
Introduction
When using seed-based methods to analyze resting state fMRI (rs-fMRI) data, the selection of the seed has large influence on the results. In previous study, we developed a method to automatically compute the seed location out of voxel clusters generated by self-organizing map (SOM) whose input was feature vectors formed by combining anatomical and rs-fMRI data[1]. In this study, we relaxed the requirement of seed at a single anatomical location in the previous study. Instead, we used most correlated voxels in each cluster to compute rs-fMRI maps. We generated candidate connectivity maps from top clusters ranked by an entropy type measure formed from cluster size and average internal correlation. The whole analysis pipeline was automatic except that the final connectivity map and “seeding cluster” were manually picked from the candidates.Methods
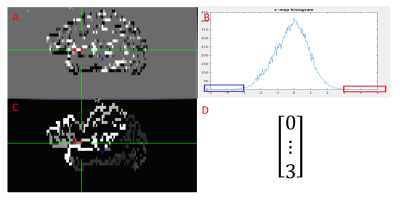
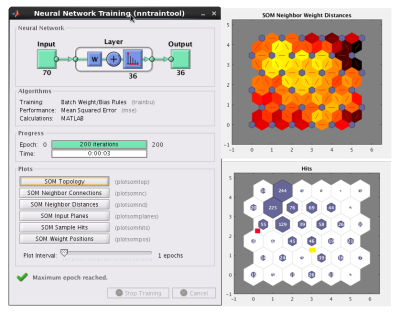
Data acquisition: Ten subjects consisting five healthy controls and five patients were scanned in an IRB-approved protocol at 3T Siemens scanner (Erlangen, Germany) using a bitebar to reduce head motion, in a 12-ch receive head coil. Scans included T1-MPRAGE (voxel size=1x1x1.2mm, matrix size=256x256x120, TE/TR/TI=1.75/1900/900ms), unilateral finger tapping fMRI( voxel size=2x2x4mm, matrix size=128x128x31, TR/TE/FA=2800/29/80, 160 volumes), and rs-fMRI(voxel size=2x2x4mm, matrix size=128x128x31, TR/TE/FA=2800/29/80, 132volumes).Data processing: Each fMRI and rs-fMRI dataset was motion-corrected, low-pass filtered and spatially filtered. T1 image was parcellated into ROIs by using FreeSurfer[2]. The ROIs were registered to rs-fMRI image space by using AFNI[3]. The two ROIs covering the motor cortex were used as searching region. From rs-fMRI data, the global connectivity between each voxel in the searching region and all other brain cortex voxels were computed and then the connectivity distribution was fitted into a Gaussian distribution[4]. The feature vectors were formed by counting the number of voxels whose connectivity value was outside three standard deviation, in the parcellated cortex ROIs. The above feature vector forming step is shown in figure 1. The feature vectors of all the voxels in the searching region was feed into a size 6x6 SOM classifier in Matlab. Using correlation among voxels in the same classified cluster as the criteria, the size of cluster was trimmed by half and the remaining voxels were all considered as seed. The connectivity map of a cluster was the average of the connectivity maps computed for all the seeds in the cluster.
Student-t maps were calculated from fMRI finger-typing data and then registered to rs-fMRI space by using AFNI.
Results
Figure 2 demonstrates the SOM classifier and the output.In all subjects, reviewer was able to find a good match to the fMRI map out of the generated seed clusters and associated connectivity maps from rs-fMRI data.
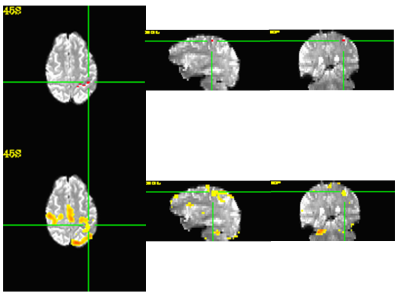
Figure 3 shows the structure of a seed cluster matching very well with the anatomical structure.
Discussion and Conclusion
We have developed a fully automatic data-driven pipeline to generate seed clusters and corresponding connectivity maps for rs-fMRI data analysis by using unsupervised machine learning method. It only needs manual participation in the very end to review the candidate seed cluster locations and connectivity maps to make decision. In contrast to common anatomical seed scheme which usually consists of a small neighborhood surrounding a single voxel, seeds in our pipeline were determined functionally within large pre-defined searching region which can be derived by using automatic brain segmentation tools like FreeSurfer. The structure of functionally determined seed cluster may demonstrate a good matching to the anatomical structure. The pipeline was tested successfully on rs-fMRI studies with accompanied task-based fMRI involving motor cortex. We will test the pipeline on more brain regions in the future.The FreeSurfer parcellation results have decisive influence on seed searching region. Propagation of a template ROI to individual subjects through image registration can be an alternative way to establish searching ROI. The FreeSurfer parcellation results also influence the generation of feature vectors.
Acknowledgements
This work was supported by the Imaging Institute, Cleveland Clinic.
Authors acknowledge technical support by Siemens Medical Solutions.
References
[1]Li M, et al. Automatic seed selection for resting state fMRI data analysis by using machine learning, ISMRM 27th Annual Meeting & Exhibition, Montreal, Canada, May 2019
[2] Jenkinson M, et al. FSL, NeuroImage.2012; 62(2):782-790.
[3] Cox RW. AFNI: What a long strange trip it’s been, NeuroImage. 2012; 62(2):743-747.
[4] Lowe MJ, et al. Functional Connectivity in Single and Multislice Echoplanar Imaging Using Resting-State Fluctuations, NeuroImage. 1998; 7(1):119-132.
Figures