3982
Dynamic Changes of the Functional Connectivity in the Default Mode Network across the Spectrum of Alzheimer’s Disease1Bioengineering, University of Washington, Seattle, WA, United States, 2Computer Science, University of Washington, Seattle, WA, United States, 3Radiology, University of Washington, Seattle, WA, United States
Synopsis
Default Mode Network functional connectivity (DMN FC) has been proposed as a non-invasive biomarker for Alzheimer’s Disease (AD). However, the dynamic relationships within the DMN over the course of the disease have not been established. We explored the dynamic FC between the DMN nodes in healthy control, mild cognitive impairment (MCI), and AD subjects using a sliding window analysis of ultrafast resting state fMRI (rs-fMRI) data. Group comparisons revealed significant trends in the dynamic measures of functional connectivity within the DMN across the spectrum of AD, suggesting compensatory systems at work within the DMN as AD progresses.
Introduction
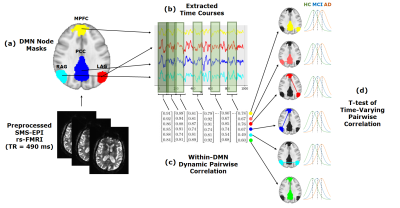
Resting state functional connectivity (FC) in the Default Mode Network (DMN) has been shown to be altered in Alzheimer’s disease (AD)1-4. However, the changes within the DMN over the course of the disease are unknown. Enabled by ultrafast resting state fMRI (rs-fMRI) data acquisition, we probed the dynamic aspects of the changes in node-to-node functional connectivity within the DMN across the spectrum of the disease. Using a sliding window analysis, we studied the DMN functional connectivity in a cohort of healthy control (HC), mild cognitive impairment (MCI), and AD subjects and characterized the trajectories of change (Figure 1). Our results indicate significant dynamic connectivity changes within the DMN over the course of the disease.Methods
48 subjects (24 HC (14 females, age = 71.8 ± 7.39), 15 MCI (5 females, age = 72.5 ± 6.37), 9 AD (3 females, age = 68.3 ± 9.66)) were scanned on a 3T (GE Discovery MR750) scanner using a 32-channel head coil (NOVA Medical). Diagnosis was assigned following neuropsychological assessment using Montreal Cognitive Assessment, Boston Naming Test and Trails during consensus meetings. Ultrafast rs-fMRI data was acquired using a SMS-EPI sequence5 with an acceleration factor of 5, CAIPI shift of FOV /3, TR/TE= 490/30 ms, scan duration = 10 min, voxel size = 3.14 x 3.14 x 4 mm6. Images were preprocessed by skull stripping, despiking, motion correction, normalization to MNI space, regression of the global signal and movement parameters, blurring of EPI volumes to a 5 mm fwhm, and application of a band pass filter of [0.01 - 0.25] Hz to remove respiratory and cardiac frequency components. In this study, we focused on the following DMN nodes: posterior cingulate cortex (PCC), medial prefrontal cortex (MPFC), left angular gyrus (LAG), and right angular gyrus (RAG). We created a mask for each of these nodes by averaging the individual DMN maps created using a standard seed-based analysis across all subjects. Using these masks, the corresponding time series were extracted and the pairwise node-to-node correlation coefficients were calculated over a sliding window (length: 120 TR = 58.8 s, overlap: 80%). For each node-to-node connection, we performed a group comparison between the collective correlation coefficient measures using two-sample unequal variance t-tests of means (Figure 1).Results
After evaluating the quality of dynamic connectivity maps generated using different window lengths, we determined that a 120 TR window length provided sufficient sensitivity in mapping the DMN at the single-subject level (Figure 2). Dynamic functional connectivity analysis results indicated: 1) between HC and MCI, the LAG/MPFC and MPFC/RAG connectivity strengths decreased 2) between MCI and AD, the connectivity strengths increased for the MPFC/RAG and LAG/RAG connections and decreased for the LAG/PCC and PCC/MPFC connections (Figures 3 and 4). To evaluate the sensitivity of the analysis to the choice of window length, we repeated the analysis using a range of window lengths varying between 40-200 TRs. Although increasing window lengths resulted in slight upward shifts in the mean correlation between nodes, the identified trends in mean correlation changes were relatively stable and insensitive to changes in window length (Figure 5). We also repeated the study using standard stationary analysis (results not shown here) and while the same trends were observed, the changes in connections were less significant.Discussion
Previous studies have indicated that PCC is one of the earlier regions affected by AD pathology7. The initial decreases between HC and MCI subjects in the LAG/MPFC and MPFC/RAG connectivity may indicate compensatory effects within the DMN to maintain the connectivity with PCC despite its pathological changes. However, between MCI and AD, the changes in connectivity occur in the opposite direction, and connections to PCC are decreased while the increase in the LAG/RAG connection brings the mean correlation to values comparable to those for HC (Figure 4). This potentially indicates further deterioration of the PCC and a shift from local to more remote or global mechanisms of compensation, which has been noted in a previous study using network centrality analysis8. However, the PCC/RAG connection remains at a relatively stable mean correlation while the MPFC/RAG connection significantly increases, even in comparison between HC and AD, suggesting that some local mechanisms of compensation may persist. When the study was repeated using standard stationary analysis, the same trends in changes in connectivity were observed, but the changes were not as significant. This suggests that dynamic functional connectivity is a more sensitive and informative biomarker than stationary functional connectivity for identifying cognitive impairment in AD. Due to the small size of our cohort, however, our findings need to be confirmed using a larger cohort.Conclusion
This preliminary study suggests that functional changes occur within the DMN throughout the progression of Alzheimer's Disease and that dynamic analysis of DMN functional connectivity using ultrafast rs-fMRI and a sliding window analysis may be a sensitive tool to differentiate between different stages of the disease.Acknowledgements
This work is supported by the University of Washington Alzheimer’s Disease Research center pilot project award and NIH grant P50 AG047366.References
1. Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637-42. doi: 10.1073/pnas.0308627101. PubMed PMID: 15070770; PMCID: PMC384799.
2. Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager AM. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2007;104(47):18760-5. doi: 10.1073/pnas.0708803104. PubMed PMID: 18003904; PMCID: PMC2141850.
3. Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, Wu T, Jiang T, Li K. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. Neuroimage. 2006;31(2):496-504. doi: 10.1016/j.neuroimage.2005.12.033. PubMed PMID: 16473024.
4. Zhang HY, Wang SJ, Xing J, Liu B, Ma ZL, Yang M, Zhang ZJ, Teng GJ. Detection of PCC functional connectivity characteristics in resting-state fMRI in mild Alzheimer's disease. Behav Brain Res. 2009;197(1):103-8. doi: 10.1016/j.bbr.2008.08.012. PubMed PMID: 18786570.
5. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. 2012. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn. Reson. Med. 2012;67:1210–1224.
6. Jahanian H, Holdsworth S, Christen T, Wu H, Zhu K, Kerr AB, Middione MJ, Dougherty RF, Moseley M, Zaharchuk G. Advantages of short repetition time resting-state functional MRI enabled by simultaneous multi-slice imaging. J Neurosci Methods. 2018;311:122-32. doi: 10.1016/j.jneumeth.2018.09.033. PubMed PMID: 30300699.
7. Choo IH, Lee DY, Oh JS, Lee JS, Lee DS, Song IC, Youn JC, Kim SG, Kim KW, Jhoo JH, Woo JI. Posterior cingulate cortex atrophy and regional cingulum disruption in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2010;31(5):772-9. doi: 10.1016/j.neurobiolaging.2008.06.015. PMID: 18687503.
8. Wang Z, Qiao K, Chen G, Sui D, Dong HM, Wang YS, Li HJ, Lu J, Zuo XN, Han Y. Functional Connectivity Changes Across the Spectrum of Subjective Cognitive Decline, Amnestic Mild Cognitive Impairment and Alzheimer's Disease. Front Neuroinform. 2019;13:26. doi: 10.3389/fninf.2019.00026. PMID: 31105548.
Figures