3977
Functional Connectivity in Glioma patients using Resting State fMRI: Effect on Default Mode, Fronto-Parietal and Dorsal Attention Networks1Department of Radiology, New York University School of Medicine, New York, NY, United States, 2Department of Neurosurgery, New York University School of Medicine, New York, NY, United States
Synopsis
Exploration of functional connectivity in patients with brain tumors with Resting state functional MRI (rsfMRI) is expanding, but methodology used and results in previous studies are heterogenous. We explored the disruption of the functional connectivity of the Default Mode, Dorsal Attention and Fronto-Parietal Networks in 35 glioma patients, with a standardized method, using both Seed-Based Connectivity Analysis and Independent Component Analysis. The Default Mode was the most affected network, with global increased connectivity contrasting with a decreased connectivity in the corpus callosum. No difference in connectivity was found between IDH mutant or wildtype tumors.
Synopsis
Exploration of functional connectivity in patients with brain tumors with Resting state functional MRI (rsfMRI) is expanding, but methodology used and results in previous studies are heterogenous. We explored the disruption of the functional connectivity of the Default Mode, Dorsal Attention and Fronto-Parietal Networks in 35 glioma patients, with a standardized method, using both Seed-Based Connectivity Analysis and Independent Component Analysis. The Default Mode was the most affected network, with global increased connectivity contrasting with a decreased connectivity in the corpus callosum. No difference in connectivity was found between IDH mutant or wildtype tumors.Introduction
Gliomas are the most frequent primitive brain tumors. Resting state functional magnetic resonance imaging (rsfMRI) is an emerging tool to explore the functional networks and disruption of normal functional connectivity in patients with brain tumors [1]. The Default Mode Network (DMN), a task-negative network, is the most prominent system in the resting state, while the Fronto-Parietal Network (FPN) and Dorsal Attention Network (DAN) play a central role in executive control and attention |2]. Previous studies described a decrease in global functional connectivity strength in patients with gliomas but analyses and results were heterogeneous [3]. In our study, we explored the relationship of DMN, FPN and DAN in glioma patients using rsfMRI in a standardized way.Methods
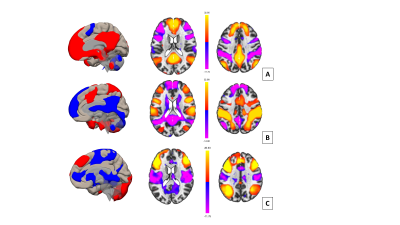
rsfMRI data of 35 patients with treatment-naïve gliomas (2015-2019, 17 WHO grade I-II, 18 grade III-IV; age: 44.6±18.5 years; 18 females, 17 males) and 70 age-matched controls from the 1000 Functional Connectomes Project [4] were analyzed using Conn functional connectivity toolbox [5]. Since the rsfMRI methodology is not standardized in the literature (different type of analysis, number of components and statistical threshold used) [6], we performed both Seed Based Connectivity Analysis (SBCA) with seeds from the software atlas and Independent Component Analysis (ICA) with different number of independent components (10 to 100 ICs) and different statistical parameters (cluster-extent FDR-corrected threshold: p<0.05; voxel-extent uncorrected threshold: p<0.001 and FDR-corrected threshold: p<0.05) to study reliably the DMN, FPN and DAN (Figure 1).Results
We detected an increase of global connectivity in the DMN of glioma patients compared to controls, with consistency using 4 seeds and different number of ICs (Figure 2) (Cluster 1: 663 voxels including the subcallosal cortex, height: p<10-6; Cluster 2: 615 voxels including the Precuneus and Posterior Cingulate Gyrus, height: p<10-5), as shown in Figure 3A. However, an area of decreased connectivity was found in the posterior corpus callosum (Cluster 1: 358 voxels corresponding to the corpus callosum, height: p<10-5), as shown in Figure 3B. This decreased connectivity was more pronounced when comparing high-grade tumors with controls. Small areas of increased connectivity in patients in the occipital region (multiple clusters with height: p<10-6) were detected in DAN. For the FPN, increased connectivity was noted in the precuneus, posterior cingulate gyrus and frontal cortex (regions of the DMN). No difference in the connectivity of the networks of interest (for both SBCA and ICA) was found between low- and high-grade tumors as well as pertaining to their IDH (isocitrate dehydrogenase) molecular status.Conclusion
We found disrupted functional connectivity in the three networks of interest in glioma patients, the most affected network being the DMN with global increased connectivity, suggesting a global dysregulation of brain connectivity due to gliomas. This could be explained by decreased connectivity between the cerebral hemispheres across the corpus callosum, in particular in patients with high-grade tumors. These results are consistent with the Wallerian degeneration within the corpus callosum explored with Diffusion Tensor Imaging in patients with glioblastoma [7]. This decreased connectivity in the corpus callosum could be a novel biomarker of the alteration of functional connectivity during the follow-up of patients with gliomas. Increased connectivity in the regions of the DMN when exploring the FPN could be related to a decreased inhibition of this executive network by the DMN due to brain tumors. Finally, no difference was found in this study between the connectivity of IDH mutant and wildtype tumors but further investigations with larger cohort are required.Acknowledgements
No acknowledgement found.References
1. Lee MH, Smyser CD, Shimony JS. Resting state fMRI: A review of methods and clinical applications. AJNR Am J Neuroradiol 2013;34:1866–72.
2. Dixon ML, Vega ADL, Mills C, et al. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. PNAS 2018;115:E1598–607.
3. Fox ME, King TZ. Functional Connectivity in Adult Brain Tumor Patients: A Systematic Review. Brain Connect 2018;8:381–97.
4. Mennes M, Biswal BB, Castellanos FX, et al. Making data sharing work: The FCP/INDI experience. NeuroImage 2013;82:683–91.
5. Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012;2:125–41.
6. Kelly RE, Wang Z, Alexopoulos GS, et al. Hybrid ICA-Seed-Based Methods for fMRI Functional Connectivity Assessment: A Feasibility Study. Int J Biomed Imaging 2010;2010.
7. Saksena S, Jain R, Schultz L, et al. The Corpus Callosum Wallerian Degeneration in the Unilateral Brain Tumors: Evaluation with Diffusion Tensor Imaging (DTI). J Clin Diagn Res 2013;7:320–5.
Figures