3976
Increased Striatal Functional Connectivity is Associated with Improved Smoking Cessation Outcomes1Department of Radiology, the Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Synopsis
Most smoking cessation attempts result in failure. The striatum is critical area of reward processing, and have been repeatedly linked to nicotine addiction. Neuroimaging studies have shown that chronic smokers had altered resting-state functional connectivity (rsFC) of striatum. Here, we further investigated the different rsFC changes of striatum subsets between smokers who relapsed and those who not relapsed after smoking cessation treatment. We found that smokers who relapsed had decreased rsFC of striatum subsets, while those who not relapse had increased rsFC of striatum subsets. These novel findings suggest that increased connectivity of striatum subsets could imporve likelihood of cessation.
Purpose
To date, most smoking cessation attempts still result in failure 1. The striatum is critical area of reward processing, and have been repeatedly linked to nicotine addiction2,3. Neuroimaging studies have identified altered resting-state functional connectivity (rsFC) of striatum among smokers relative to nonsmokers4,5. However, the recovery neuromechanism underlying tobacco addiction in the rsFC network of striatum subsets remains unclear. Based on our review of previous sudies, we hypothesized that different smoking cessation outcomes (relapse or not) might be associated with different FC changes of striatum subsets after smoking cessation treatment. Therefore, by longitudinal comparisons between post- and pre-treatment smokers using rsFC, the present study investigated the different rsFC changes of striatum subsets between the smokers who relapsed to smoking and those who not relapsed after smoking cessation treatment.Methods
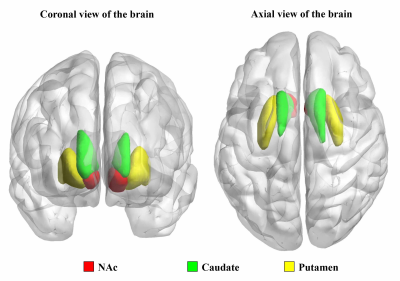
Nicotine-dependent smokers were initially recruited into the study, all of whom underwent magnetic resonance imaging (MRI) scans. After a 12-week treatment with varenicline, a first line drug for smoking cessation, the subjects who underwent an additional MRI scan were finally included in the study. Of the 30 smokers who completed the follow-up MRI scan, 14 smokers relapsed to smoking (relapsers) and 16 smokers not relapsed (non-relapsers). All the data were acquired using a 3.0 T GE Signa MR scanner. Functional image preprocessing and statistical analyses were conducted using DPABI toolbox (Yan, et al., 2016, http://rfmri.org/DPABI), which is performed on the MATLAB (The Mathworks, Inc., USA). Then, the left and right striatum subsets (including nucleus accumbens (NAc), caudate and putamen)(Fig. 1)were used as seed regions for seed-based rsFC analysis. rsFC maps were generated by calculating. Lastly, Changes in rsFC maps of each striatum subsets (left and right caudate, NAc and putamen) across groups and visits were determined using a 2 (Group: relapsers and non-relapsers) × 2 (Visit: before and after treatment) repeated measure ANCOVA with age as a confound. Then, the resulting statistical interaction maps were corrected for multiple comparisons using Gaussian random field (GRF) correction, and clusters survived two-tailed GRF correction if voxel Z ˃ 1.96, cluster-level p < 0.0083 (Bonferroni correction, p <0.05/6=0.0083).Results
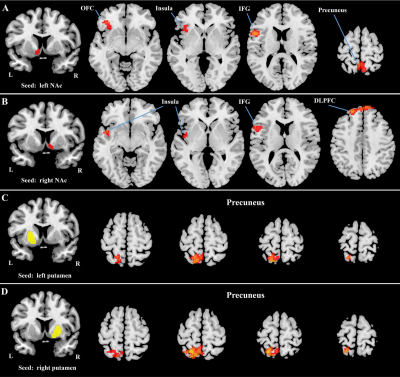
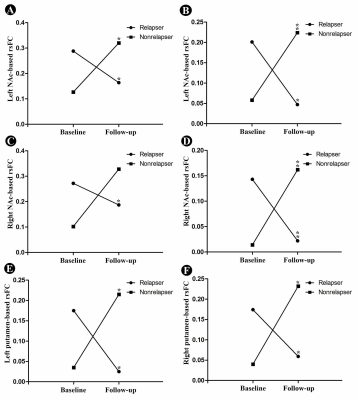
There was no significant difference in age, smoking data between the relapsers and non-relapsers. Longitudinal changes in the rsFC of each striatum subsets (left and right caudate, NAc and putamen) in both relaspers and nonrelapsers groups, and their interaction were assessed using repeated measures ANCOVA. Significant interaction effects were detected: 1) between the left NAc and left orbitofrontal cortex (OFC)/inferior frontal gyrus (IFG)/insula (cluster 1) and bilateral precuneus (cluster 2) (Figure 2A); 2) between the right NAc and left IFG/insula (cluster 1) and bilateral dorsolateral prefrontal cortex (DLPFC) (cluster 2) (Figure 2B); 3) between the left putamen and left precuneus (Figure 2C); and 4) between the right putamen and left precuneus (Figure 2D). Then, post-hoc region-of-interest analyses for the brain area showing interaction effects indicated significantly decreased rsFC in relapsers (post- vs. pre-treatment) and significantly increased rsFC in nonrelapsers (post- vs. pre-treatment) (Figure 3A-3F).Discussion and Conclusion
This longitudinal study used resting-state fMRI to compare rsFC changes of striatum subsets during smoking cessation treatment using varenicline among smokers with different treatment outcomes (relapse or not). For NAc seeds, significant decreases of rsFC were observed with several regions, including OFC, DLPFC, IFG, insula, and precuneus among relapsers; for putamen seeds, significant decreases of rsFC were observed with left precuneus; additionally, the opposite pattern was observed among nonrelapsers. To our knowledge, this is the first study to explore the recovery neuromechanism of nicotine addiction by investigating rsFC of the striatum. Our findings showed that increased rsFC of the striatum subsets (NAc and putamen) could help resist relapse to improve smoking cessation outcomes. That’s to say, individuals with increased rsFC of the striatum subsets (NAc and putamen) during smoking cessation treatment would more likely quit smoking successfully. These striatum subsets circuits may serve as potential therapeutic targets for more efficacious treatment of nicotine addiction.Acknowledgements
CW was supported by zhejiang provincial natural science foundation (LQ18H180001) and Medical and Health Scientific Research Fund Project of Zhejiang Province (2017KY080). MZ was supported by the Natural Science Foundation of China Surface Project (81171310).References
1. Cahill K, Stead LF, Lancaster T . Nicotine receptor partial agonists for smoking cessation. The Cochrane database of systematic reviews. 2010; (12): CD006103.
2. O'Doherty J, Dayan P, Schultz J et al. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004; 304: 452-454.
3. David SP, Munafo MR, Johansen-Berg H et al. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry. 2005; 58: 488-494.
4. Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012; 62: 2281-2295.
5. Hong LE, Gu H, Yang Y et al. Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009; 66: 431-441.
Figures