3969
Connectome predictive modeling of face-name associations in older adults with mild cognitive impairment1Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States, 2Functional MRI Laboratory, University of Michigan, Ann Arbor, MI, United States, 3Psychiatry, University of Michigan, Ann Arbor, MI, United States, 4Mental Health Service, VA Ann Arbor Healthcare System, Ann Arbor, MI, United States
Synopsis
Connectome predictive modeling was applied to task data. Leveraging a MCI-relevant face-name task illuminates relationships with measures of total cognition (RBANS) and memory (Free Recall). This provides support for the use of clinically-relevant tasks in cases where a “driven” connectivity network may elucidate pathological changes.
Introduction
Functional connectivity has demonstrated utility in identifying pathological brain network changes, such as connectivity differences during memory encoding and retrieval that indicate loss of cognitive control.1 A recent analysis technique, connectome predictive modeling (CPM), successfully relates behavioral outcomes and memory integrity to resting-state fMRI connectivity measures.2-5 CPM applied to task-based fMRI has provided additional insight into cognitive abilities and clinical diagnoses, potentially due to the driven network connectivity.6,7 Furthermore, connectome predictive analysis that leverages a task designed to mimic real-world challenges faced by those with mild cognitive impairment (MCI) such as object location association has been shown to have additional utility in predicting behavior in this population.8 Similarly, face-name associations emulate common real-world memory complaints by those with MCI. Previous work discerned a loss of memory encoding and retrieval related to the hippocampus and lateral frontoparietal regions, respectively, in patients with MCI compared to healthy older adults.9 Here, we applied CPM to evaluate the relevant face-name functional task in terms of behavioral prediction capabilities.Methods
Functional data were acquired for 45 subjects with MCI (age=72.8±8.2, 28M/17F) on a 3T MR750 GE scanner using multiband EPI (MB factor=3). Functional scan parameters were: TR: 1200 ms, TE: 30 ms, FOV: 220 mm, 51 axial slices, and 2.5 mm isotropic voxels.The face-name task was comprised of 2 functional runs (6 min, 20 s each) using a mixed event-related block design. Five stimuli were shown for 5 seconds each during six active blocks, with each stimulus separated by an interstimulus interval of 1, 2, 3, 4, or 5 seconds, resulting in total durations of 34-46 seconds for each active block.10 In this analysis, only the first run was analyzed for each subject. Behavioral measures of interest included free recall from a pre-scan object location association test (the distance, in cm, between the location selected from memory without the associated contextual information and actual location)10 and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) Total Score.
The following preprocessing steps were applied to the imaging data: cardiac and respiratory noise correction (RETROICOR11), slice timing correction (SPM8), and image registration (FSL). A framewise displacement threshold12 of 0.5 was applied to determine frames between which to smooth.13 Structural and functional images were co-registered (SPM8). Structural images were normalized into MNI space (VBM8) and the resulting normalization matrix was applied to functional data.
Functional data were parcellated into 264 regions of interest (ROIs) using the Power atlas,14 with 8 additional ROIs selected from the amygdala and hippocampus region for a total of 272 ROIs. Average time courses in these ROIs were correlated with all other voxels of the brain (Pearson product-moment correlation). Z-scores were computed from these correlation matrices using a Fisher transformation. Input connectivity data and behavioral measures were orthogonalized to age.
CPM was implemented according to the protocol in Shen.4 Connectivity matrices were thresholded at 0.01 and this mask was applied to the original connectivity values to be summed into the overall brain score. This process is done for positive and negative correlations individually. Then, a model is fit under the assumption of a linear relationship between brain score and behavioral score. A leave-one-out framework was used, with the “left-out” subject used to test the fit linear model. Permutation testing was done with 1000 iterations to evaluate the significance of fits.
Significant model connections were visualized with the Yale BioImage Suite, to evaluate lobule connections across hemispheres, and to display important nodes (defined by degree of connectedness, thresholded to look at significant connections from the top 5 nodes for each model).
Results
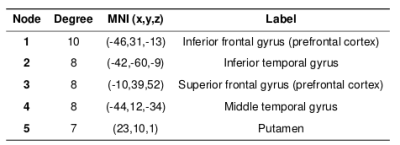
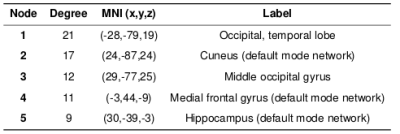
For the face-name task, significant positive relationships were found between CPM scores and Object-Location Free Recall (R=0.38, p=0.0099), and between CPM brain scores and RBANS (R=0.31, p=0.042), shown in Figures 1 and 2. The highest degree nodes for Free Recall are centralized in prefrontal and default mode regions, while highest degree nodes for RBANS correspond to visual and default mode regions, as shown in Tables 1 and 2.Discussion
CPM is able to successfully predict behavioral performance from task fMRI data for MCI subjects using a face-name task. This work found significant relationships for measures of overall cognition (RBANS) and independent measures of memory (Object-Location Free Recall). These positive relationships indicate that improved performance corresponds to increased connectivity (positive edges) or reduced connectivity (negative edges). High degree nodes located in the prefrontal regions may be indicative of compensatory-related increased activity in these regions in those with MCI.15-17 Similarly, the default mode network is linked to memory and patients at risk of AD have been shown to have weakened task-induced deactivation of the DMN.18 Future work will examine applying CPM to other datasets such as those of the Alzheimer’s Disease Neuroimaging Initiative (ADNI) and combining multiple modalities (i.e., resting-state and task-based fMRI) for CPM analysis.Acknowledgements
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and Rehabilitation Research and Development Service (IRX001534) and by the Michigan Alzheimer’s Disease Center (NIA: 5P30AG053760-5).References
1. Hampstead BM, Khoshnoodi M, Yan W, Deshpande G, Sathian K. Patterns of effective connectivity during memory encoding and retrieval differ between patients with mild cognitive impairment and healthy older adults. NeuroImage. 2016;124(Part A):997-1008.
2. Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, Papademetris X, Constable RT. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nature neuroscience. 2015,18:1664.
3. Lake EM, Finn ES, Noble SM, Vanderwal T, Shen X, Rosenberg MD, Spann MN, Chun MM, Scheinost D, Constable RT. The functional brain organization of an individual predicts measures of social abilities in autism spectrum disorder. BioRxiv. 2018, 290320.
4. Shen X, Finn ES, Scheinost D, Rosenberg MD, Chun MM, Papademetris X, Constable RT. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nature protocols. 2017, 12:506.
5. Peltier S, Ma S, Giordani B, Paulson H, Snyder R, Hampstead B. Predicting memory impairment using resting-state brain connectomes in older adults. In Proceedings of the 27th Annual Meeting of the ISMRM, Montreal, QC, Canada. 2019, p. 0881.
6. Rosenberg MD, Finn ES, Scheinost D, Papademetris X, Shen X, Constable RT, Chun MM. A neuromarker of sustained attention from whole-brain functional connectivity. Nature neuroscience. 2016, 19:165.
7. Greene AS, Gao S, Scheinost D, Constable RT. Task-induced brain state manipulation improves prediction of individual traits. Nature communications. 2018 Jul 18;9(1):2807.
8. Peltier S, Ma S, Moll A, Laing J, Hampstead B. Connectome predictive modeling in subjects with mild cognitive impairment: Resting-state and object location task results. In Proceedings of the 27th Annual Meeting of the ISMRM, Montreal, QC, Canada, 2019. p. 3282.
9. Hampstead BM, Stringer AY, Stilla RF, Amaraneni A, and Sathian K. Where did I put that? Patients with amnestic mild cognitive impairment demonstrate widespread reductions in activity during the encoding of ecologically relevant object-location associations. Neuropsychologia. 2011; 49: pp. 2349-2361.
10. Hampstead BM, Sathian K, Bikson M, Stringer AY. Combined Mnemonic Strategy Training and High Definition Transcranial Direct Current Stimulation for Memory Deficits in Mild Cognitive Impairment Alzheimer's & Dementia. Translational Research & Clinical Interventions. 2017, 3:459.
11. Glover GH, Li TQ, Ress D. Image‐based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magnetic Resonance in Medicine. 2000, 44:162.
12. Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014, 84:320.
13. Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. Impact of in‐scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. NeuroImage. 2012, 60:623.
14. Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron. 2011, 72:665.
15. Rosano C, Aizenstein HJ, Cochran JL, Saxton JA, De Kosky ST, Newman AB, Kuller LH, Lopez OL, Carter CS. Event-related functional magnetic resonance imaging investigation of executive control in very old individuals with mild cognitive impairment. Biol. Psychiatry. 2005, 57 (7) , pp. 761-767.
16. Gigi A, Babai R, Penker A, Hendler T, Korczyn AD. Prefrontal Compensatory Mechanism May Enable Normal Semantic Memory Performance in Mild Cognitive Impairment (MCI). Journal of Neuroimaging. 2010, 20: 163-168.
17. Heun R, Freymann K, Erb M, Leube DT, Jessen F, Kircher TT, Grodd W. Mild cognitive impairment (MCI) and actual retrieval performance affect cerebral activation in the elderly. Neurobiol Aging. 2007, 28: 404-413.
18. Pihlajamäki M, Sperling RA. Functional MRI Assessment of Task-Induced Deactivation of the Default Mode Network in Alzheimer’s Disease and At-Risk Older Individuals. Behavioural Neurology. 2009, 21(1-2): 77-91.
Figures
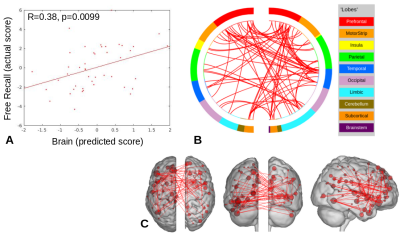
Figure 1. Object-Location Free Recall CPM results for face-name task data (positive edges).
A) Plot of predicted versus actual Free Recall values.
B) Circle plot of significant connections between brain areas (using node degree threshold of 7).
C) Glass brain plot of significant nodes and connections.
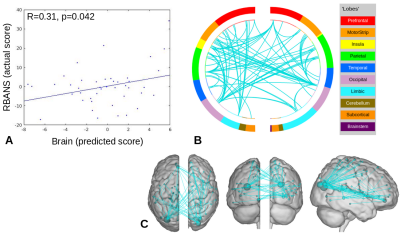
Figure 2. RBANS CPM results for face-name task data (negative edges).
A) Plot of predicted versus actual RBANS values.
B) Circle plot of significant connections between brain areas (using node degree threshold of 9).
C) Glass brain plot of significant nodes and connections.