3959
Physiologic noise contributes to large scale switching in PCC-seeded spontaneous co-activation1Radiology, Cleveland Clinic, Cleveland, OH, United States
Synopsis
We investigated the impact of physiologic noise correction on PCC seeded co-activation pattern (CAP) analysis varying the cluster number. We found that patterns from PCC seeded CAP analysis were best classified as 5 sub-patterns of default mode, sensory visual and motor, salience, central executive networks and noise. Also we observed that physiologic noise correction resulted in less frequent salience and central executive networks from PCC-related co-activations than uncorrected data.
Introduction
The posterior cingulate cortex (PCC) is an important brain region for resting state functional connectivity, being a central node in the default mode network1. This brain region is known to be especially susceptible to physiologic noise effects2. Recently, the spontaneous co-activation (CAP) approach has shown potential to investigate dynamic changes in resting state fMRI3,4. However, CAP analysis results will vary depending on pre-defined number of clusters. In this study, we examine CAP analysis results varying cluster number and investigate physiologic noise correction effects on PCC seeded CAP analysis.Methods
Twenty eight healthy controls were scanned at 3T using single band EPI with pulse plethysmograph and respiratory belt recording (TR=2.8s, 128x128 matrix, 31 slices, 132 repetitions). In order to focus strictly on the impact of physiologic noise correction on CAP results, we employed exactly the processing pipeline of Liu et al.3. For the physiologic noise analysis, RETROICOR5 and RVHR6 were applied at the beginning of the pipeline.CAP analysis
The seed, PCC (MNI, [0,-53,26]) related spontaneous CAP were calculated using kmeans (100,000 iterations) with 4 to 9 cluster number. To improve SNR, the time frames at the top 15% of signals were selected and the mask of the top 10% and the bottom 5% of voxels were chosen before k-mean clustering as described3. The classified patterns are sorted from the largest to the smallest fraction4.
CAP analysis varying clustering number
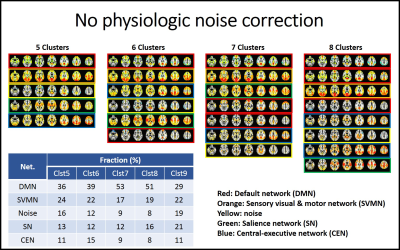
As the cluster number is increased from 4 to 9, classified patterns were visually inspected. In the analysis without physiologic noise correction, we found that the CAP analysis with 5 clusters resulted in patterns that can be classified as default mode (DMN), sensory visual and motor sensory network (SVMN), salience network (SN), and central executive networks (CEN) and noise. CAP analyses with higher cluster numbers appear to break these 5 patterns into sub-patterns, which we classified based on which of the 5 cluster CAP patterns the sub-pattern had the highest spatial correlation with. We then repeated this process with the physiologic noise corrected data.
Results
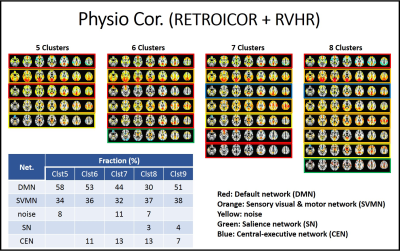
Fig1 and 2 show CAP pattern from 5 to 8 clusters with uncorrected data and physiologic noise corrected data, respectively. The corresponding factions of 5 to 9 cluster CAP analysis are presented in embedded tables.We found that the major impact of physiologic noise correction was to reduce the fraction of SN and CEN detected patterns in the CAP analysis.
Discussion
It has been reported that large scale DMN, SN and CEN switch dynamically during rest7, 8. While has been suggested that that SN controls DMN and CEN, based on Granger causality analysis7, its interaction and pattern are not known. Our study indicates that PCC seeded CAP analysis reveals DMN, SN and CEN patterns, and that removal of physiologic noise reduced the frequency of SN and CEN patterns. It might be explained that the arousal depth is reflected on physiologic condition 9, 10 and this physiologic change could be related to the switching of PCC-related co-activations between DMN, SN and CEN.Acknowledgements
Authors appreciate Dr. Xiao Liu for sharing co-activation analysis script.References
1. Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America 2004;101:4637-4642.
2. Birn RM, Murphy K, Bandettini PA. The effect of respiration variations on independent component analysis results of resting state functional connectivity. Human brain mapping 2008;29:740-750.
3. Liu X, Duyn JH. Time-varying functional network information extracted from brief instances of spontaneous brain activity. Proceedings of the National Academy of Sciences of the United States of America 2013;110:4392-4397.
4. Chen JE, Chang C, Greicius MD, Glover GH. Introducing co-activation pattern metrics to quantify spontaneous brain network dynamics. Neuroimage 2015;111:476-488.
5. Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 2000;44:162-167.
6. Chang C, Cunningham JP, Glover GH. Influence of heart rate on the BOLD signal: the cardiac response function. Neuroimage 2009;44:857-869.
7. Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America 2008;105:12569-12574.
8. Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci 2010;14:277-290.
9. Chang C, Leopold DA, Scholvinck ML, et al. Tracking brain arousal fluctuations with fMRI. Proceedings of the National Academy of Sciences of the United States of America 2016;113:4518-4523.
10. Liu X, de Zwart JA, Scholvinck ML, et al. Subcortical evidence for a contribution of arousal to fMRI studies of brain activity. Nat Commun 2018;9:395.
Figures