3958
Brain mechanism of anesthesia and sedation: fMRI functional connectivity study with minimized impact of physiological background noise in rats1Emory University/Georgia Institute of Technology, Atlanta, GA, United States
Synopsis
Different anesthetic states and sedation may have varying contributions to BOLD from physiological background noise that may challenge a comparison across brain states. Little is known on brain functional organization between anesthesia and sedation. We used a scan strategy optimized to minimize impact from physiological background noise to examine the effects of different anesthetics on functional connectivity (FC). Our results demonstrate distinct FC patterns across anesthesia depth transition and sedation, providing insight into potential brain mechanisms of unconsciousness that include global modulation of functional connectivity by isoflurane, and distant functional connectivity impairment in dexmedetomidine.
Introduction
During anesthesia or sedation, the brain is in an unconscious state. However, little is known on the underlying brain mechanisms of unconsciousness. Resting state functional MRI (rs-fMRI) has been used to study the functional organization of the whole brain under anesthesia, but one of the challenges is that most anesthetics affect physiological processes and affect functional connectivity measurements. Different anesthetic states may have varying contributions from physiological background noise[1]. We used a scan strategy optimized to minimize impact from physiological background noise to examine the effects of different anesthetics on functional connectivity. Our preliminary findings provide insight into potential brain mechanisms of unconsciousness that include global modulation of functional connectivity by isoflurane, and distant functional connectivity impairment in dexmedetomidine.Method
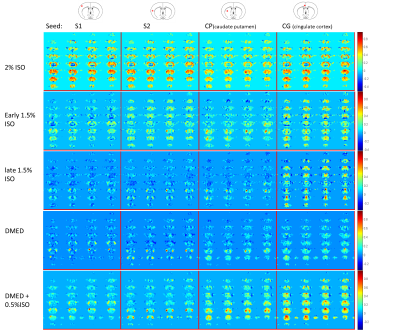
Five intubated Sprague-Dawley rats, male 300-400g, were used for the resting-state fMRI scans across conditions of 2% and 1.5% isoflurane (ISO) and dexmedetomidine (DMED). The animals were paralyzed by pancuronium and kept in normal physiological condition with fully monitoring. To minimize influence from background physiological noise, image acquisition was phase-locked to respiration to minimize variation induced by respiratory pulses. Moreover, three time points were averaged to minimize variation related to cardiac pulsation. The optimized procedure is illustrated in Fig. 1. All scans were conducted with 9.4T Bruker scanner with gradient echo EPI, TR/TE = 2459ms/15ms. A customized single loop surface coil with oval shape (2×2.5, cm) was used to fit the top of the rat brain to enhance homogeneity of the brain signal along anterior to posterior direction. For whole brain coverage, 18 axial slices (horizontal plane) with 0.6mm isotropic voxel size were used for 3 scans (TR=682ms each), which were averaged within a resulting final TR of 2459ms. The mechanical ventilation was set to 1.22Hz, i.e. 1/3 TR to sync each scan for phase-locked data acquisition. All data sets were acquired with both blip EPI and reverse blip EPI for distortion correction by FSL topup. Approximately 30 min of data (three 10 min sessions, separated by 5min intervals) of each condition was acquired under ISO (2% or 1.5%), DMED or DMED mixed with low-dose ISO (0.5%). To evaluate FCs from different functional systems, the seed-based FC analyses, including commonly-used cortical areas S1, S2 and cingulate cortex (Cg) and subcortical caudate putamen (CP), were conducted after routine pre-processing, using muscle outside the brain as a regressor instead of global signal. The correlation coefficient analysis were based on filtered time courses, <0.1 Hz in ISO and <0.2 Hz for DMED to accommodate altered vascular responses to neuronal activation[2].Results
During 2% ISO, all 5 animals show significant global correlation, regardless of the different seed regions. The thalamus shows negative correlation to the global activity pattern in 4/5 rats. The global correlation reduced gradually during the first 20min after switching to 1.5% ISO. The cingulate correlated default-mode network can be identified in 1.5% ISO. During DMED, the bilateral hemispheric correlations, especially S2 and CP, became strong but cingulate correlation of the default-mode network was reduced. During mixed DMED and 0.5% ISO, the default-mode network showed up again along with other bilateral hemispheric FCs, but global correlation increased as well. Representative data are shown in Figure 2 for demonstration.Discussion
In the present whole-brain fMRI study, the characteristic of FC patterns induced by specific anesthesia/sedation conditions could be significant, linking to the results of pharmacological action. The deep anesthesia in 2% ISO exhibits strong FC synchronization across most cortical areas, consistent with previous electrophysiological findings, i.e. large scale burst-suppression patterns[3, 4], and fMRI reports[5]. To avoid influence of global signal regression, we used surrounding head muscle signal to regress out global variation instead of whole brain. Interestingly, the brain global coactivation was reduced and mostly the distant FCs decreased when switched to 1.5% ISO, but the cingulate cortex related default-mode network was largely maintained. However, the default-mode network connectivity was largely reduced in DMED although bilateral functional networks became strong. The characterized FCs might relate to action site, e.g. DMED affects brain stem circuits[6]. The default-mode network was recovered after the addition of low-dose ISO (0.5%), with slightly increased global co-activation. The detected default-mode network regions are largely similar as previous ICA findings[7]. The reduced distant FC of default-mode network in DMED might a result of decreased norepinephrine since DMED acts on highest density of alpha-2 adrenergic receptors in locus coeruleus[8]. However increased norepinephrine has been reported in ISO[9]. Anesthesia is a challenging issue for BOLD-based studies, as it can not only alter and reduce vascular responsiveness to neuronal activation, but also introduce strong noise from elevated physiological pulse amplitude Our method improved SNR and facilitate valid comparison across studies in potentially varying noise conditions.Conclusion
The present fMRI examination optimized for minimal sensitivity to physiological cycles in rats’ multiple brain states over anesthetic depths and sedation provide a comprehensive comparison on them. The distinct functional organization between anesthesia and sedation may likely result in global activity modulation and distant disconnection respectively. Further studies will include simultaneous recording of neuronal electrophysiological activities and calcium fluorescence signals from cortical or selected deep brain sites, guided by these initial fMRI studies to facilitate selection of target sites.Acknowledgements
No acknowledgement found.References
1. Kiviniemi, V., et al., Ultra-fast magnetic resonance encephalography of physiological brain activity - Glymphatic pulsation mechanisms? J Cereb Blood Flow Metab, 2016. 36(6): p. 1033-45.
2. Pan, W.J., et al., Infraslow LFP correlates to resting-state fMRI BOLD signals. Neuroimage, 2013. 74: p. 288-97.
3. Niedermeyer, E., The burst-suppression electroencephalogram. Am J Electroneurodiagnostic Technol, 2009. 49(4): p. 333-41.
4. Ching, S., et al., A neurophysiological-metabolic model for burst suppression. Proc Natl Acad Sci U S A, 2012. 109(8): p. 3095-100.
5. Liu, X., et al., The Change of Functional Connectivity Specificity in Rats Under Various Anesthesia Levels and its Neural Origin. Brain Topogr, 2012.
6. Breton-Provencher, V. and M. Sur, Active control of arousal by a locus coeruleus GABAergic circuit. Nat Neurosci, 2019. 22(2): p. 218-228.
7. Hsu, L.M., et al., Constituents and functional implications of the rat default mode network. Proc Natl Acad Sci U S A, 2016. 113(31): p. E4541-7.
8. Zilles, K., M. Qu, and A. Schleicher, Regional distribution and heterogeneity of alpha-adrenoceptors in the rat and human central nervous system. J Hirnforsch, 1993. 34(2): p. 123-32.
9. Anzawa, N., et al., Increased noradrenaline release from rat preoptic area during and after sevoflurane and isoflurane anesthesia. Can J Anaesth, 2001. 48(5): p. 462-5.
Figures