3957
Sensory-evoked fMRI of transgenic mouse is enhanced by deafness1Center for Neuroscience Imaging Research (CNIR), Institute for Basic Science (IBS), Suwon-si, Korea, Republic of, 2Department of Health Science and Technology, Samsung Advanced Institute for Health Sciences and Technology (SAIHST), Sungkyunkwan University (SKKU), Seoul, Korea, Republic of, 3Department of Biomedical Engineering, Sungkyunkwan University (SKKU), Suwon-si, Korea, Republic of
Synopsis
BOLD fMRI is ideal for investigating neural plasticity of transgenic mouse lines. Visual and somatosensory functions can be reorganized following deafness in the early stage and adult in humans and animals. However, the extent of the neural plasticity remains unclear. To determine how deafness affects other sensory areas, 9.4T BOLD fMRI of sensory stimulation was measured before and after deafening in transgenic adult mice. The impact of scanner noise to evoked responses was also investigated in wild-type mice. Our results suggest that the sensory loss in one modality affects other sensory activities.
Introduction
Neural plasticity enhances functional responses in spared senses as a result of sensory loss in the critical period [1, 2]. However, several studies reported that this ‘cross-modal’ phenomenon is also present in the adult [3-5]. Visual deprivation leads to enhanced function of auditory cortex and deafness induces somatosensory responses in the auditory cortex. However, the neural mechanisms underlying the neural plasticity remains unclear. To address this question, we measured BOLD fMRI response of adult mice responding to visual and somatosensory stimulation, before and after deafening. Deafening can be induced by a single diphtheria toxin injection to transgenic Pou4f3DTR/+ mice [6]. Since loud scanner noise during fMRI scans can influence fMRI response [7, 8], its effect to hemodynamics was measured with CBV-weighted optical intrinsic signal imaging (OIS).Methods
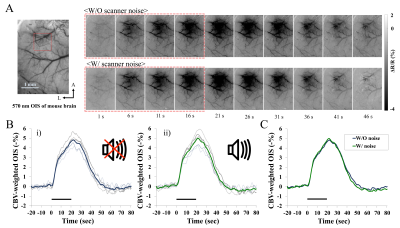
Four Pou4f3DTR/+ and three wild-type (WT) mice were used for BOLD fMRI at 9.4T, while five C57BL/6 mice were used for CBV-weighted OIS imaging. Diphtheria toxin was injected to transgenic mice, and complete hearing loss was induced about 5 days later [6]. All functional experiments were performed under ketamine and xylazine anesthesia [9]. BOLD fMRI at 9.4T (GE-EPI; TR/TE = 1000/16ms, 188x188x500µm3) was measured i) before and 7 days after deafening in the Pou4f3DTR/+ mice (n=4), and ii) in the control group (WT) for controlling repeated fMRI experiments and effects of diphtheria toxin (n=3). Bilateral visual (10ms, 5Hz and about 40lux) and unilateral forepaw stimulation (0.5ms, 4Hz and 0.5mA) was alternated. The stimulation paradigm was 40sec baseline-20sec stimulation-60sec baseline. To determine whether acoustic noise can influence evoked hemodynamic responses as seen in humans [7, 8], CBV-weighted OIS imaging responding to forepaw stimulation was performed with the MVX-10 microscope with 572±15nm filter on wild type mice (C56BL/6) with or without EPI noises (~115dB) (n=5).Results
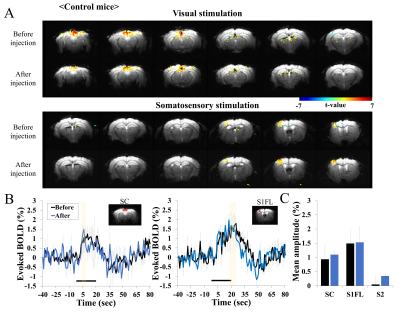
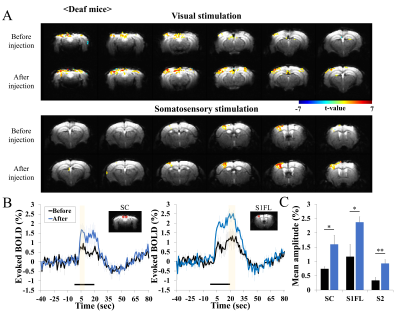
In repeated BOLD fMRI studies of control mice, reproducible activation was observed during visual and somatosensory stimulation (Fig. 1), indicating that the injection of diphtheria toxin does not impact fMRI. However, in deaf Pou4f3DTR/+ mice, enhanced BOLD response was observed during both stimuli (Fig. 2). Clearly, evoked BOLD responses in superior colliculus (SC), primary somatosensory cortex (S1FL) and secondary somatosensory cortex (S2) ROI increased after the deafness. Note that BOLD changes in other regions (e.g., V1) were not computed due to low sensitivities. The evoked BOLD fMRI difference in hearing vs. deaf mice can be due to the high intensity acoustic EPI noise. Thus, evoked CBV response of mouse S1 was measured without and with scanner noise, and found no difference of evoked OIS responses (Fig. 3). This demonstrates that the observed difference of fMRI data in hearing vs. deaf mice is not related to EPI noises.Discussion and Conclusion
In our data, EPI acoustic noise does not impact hemodynamic responses. For our fMRI protocol, the RMS level of the scanner noise was about 115dB and the peak of the power spectral density occurred at 1.3kHz. Since the mouse hearing range covers a relatively high-frequency (2kHz to 100kHz in mice vs. 20 Hz to 20 kHz in humans), the low frequency EPI noise relative to the mouse hearing frequency does not activate auditory neural networks [10, 11]. The enhanced functional activities in the somatosensory and visual areas after deafening may be due to cross-modal plasticity, or due to deprivation of cross-modal interactions between auditory and other modalities. Previous studies showed that a week of visual deprivation induced a reorganization of other sensory networks in post-critical-period adults [4, 12], due to strengthening of thalamocortical connections [4, 13]. Similar fMRI studies at ultrahigh fields such as 15.2T can be used for detecting thalamic activity [14], and thalamocortical strength can be estimated [13]. Another possible explanation is that the modification of ongoing activities may change baseline spontaneous activities, which will modulate evoked responses. This can be examined by investigating cross-modal effects with optogenetic or chemogenetic tools. In hearing mice, fMRI of somatosensory or visual stimulation can be performed without and with silencing auditory circuits. Then, the contribution of cross-modal interaction to evoked sensory responses can be determined. Further systematic studies are needed.Acknowledgements
This work is supported by Institute for Basic Science (IBS-R015-D1)References
1. Bavelier, D. and H.J. Neville, Cross-modal plasticity: where and how? Nature Reviews Neuroscience, 2002. 3(6): p. 443.
2. Lomber, S.G., M.A. Meredith, and A. Kral, Cross-modal plasticity in specific auditory cortices underlies visual compensations in the deaf. Nature neuroscience, 2010. 13(11): p. 1421.
3. Allman, B.L., L.P. Keniston, and M.A. Meredith, Adult deafness induces somatosensory conversion of ferret auditory cortex. Proceedings of the National Academy of Sciences, 2009. 106(14): p. 5925-5930.
4. Petrus, E., et al., Crossmodal induction of thalamocortical potentiation leads to enhanced information processing in the auditory cortex. Neuron, 2014. 81(3): p. 664-673.
5. Petrus, E., et al., Vision loss shifts the balance of feedforward and intracortical circuits in opposite directions in mouse primary auditory and visual cortices. Journal of Neuroscience, 2015. 35(23): p. 8790-8801.
6. Tong, L., et al., Selective deletion of cochlear hair cells causes rapid age-dependent changes in spiral ganglion and cochlear nucleus neurons. Journal of Neuroscience, 2015. 35(20): p. 7878-7891.
7. Elliott, M.R., R.W. Bowtell, and P.G. Morris, The effect of scanner sound in visual, motor, and auditory functional MRI. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 1999. 41(6): p. 1230-1235.
8. Tomasi, D., et al., fMRI-acoustic noise alters brain activation during working memory tasks. Neuroimage, 2005. 27(2): p. 377-386.
9. Shim, H.-J., et al., Mouse fMRI under ketamine and xylazine anesthesia: Robust contralateral somatosensory cortex activation in response to forepaw stimulation. Neuroimage, 2018. 177: p. 30-44.
10. Turner, J.G., et al., Hearing in laboratory animals: strain differences and nonauditory effects of noise. Comparative medicine, 2005. 55(1): p. 12-23.
11. Reynolds, R.P., et al., Noise in a laboratory animal facility from the human and mouse perspectives. Journal of the American association for laboratory animal science, 2010. 49(5): p. 592-597.
12. Van Brussel, L., A. Gerits, and L. Arckens, Evidence for cross-modal plasticity in adult mouse visual cortex following monocular enucleation. Cerebral Cortex, 2011. 21(9): p. 2133-2146.
13. Yu, X., et al., Thalamocortical inputs show post-critical-period plasticity. Neuron, 2012. 74(4): p. 731-742.
14. Jung, W.B., H.-J. Shim, and S.-G. Kim, Mouse BOLD fMRI at ultrahigh field detects somatosensory networks including thalamic nuclei. NeuroImage, 2019. 195: p. 203-214.
Figures