3952
Network scale optogenetic fMRI with tapered optical fibers1Functional Neuroimaging Laboratory, Istituto Italiano di Tecnologia, Rovereto, Italy, 2Center for Biomolecular Nanotechnologies, Istituto Italiano di Tecnologia, Arnesano, Italy, 3Optical approaches to brain function, Istituto Italiano di Tecnologia, Genova, Italy, 4Dip. di Ingegneria dell’Innovazione, Università del Salento, Lecce, Italy
Synopsis
Optogenetic fMRI is an attractive tool for studying neuronal manipulation dynamics at multiple scales, but currently lacks techniques for network (i.e. mesoscale) level investigations due to the small illumination volume of traditional flat-faced optical fibers. Here we show that a recently developed tapered fiber design allowed simultaneous, artefact-free illumination of large cortico-hippocampal areas. We also demonstrated that low-frequency cortico-hippocampal entrainment resulted in a prominent functional desynchronization of the mouse default mode network. These results support the use of this technique for deconstructing network-scale substrates and probing brain-wide functional network dynamics.
Introduction
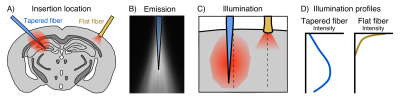
fMRI has become a fundamental tool in systems neuroscience. Despite its pervasiveness however, the relationship between regional neural activity and fMRI-based measurements of network dynamics remains unknown. In recent years, the combined use of optogenetics and fMRI (opto-fMRI) has made headway for investigating the impact of focally modulated neural populations on brain-wide patterns of functional activity1. However, these manipulations are limited in their spatial extent due to the small illumination volume of traditional flat-faced fibers; an aspect that cannot be simply overcome by using higher irradiance owing to the risk of tissue heating and spurious hemodynamic responses2. Ultimately, this limitation prevents the spatially extended manipulation of neurons required to understand the contribution of large neuronal ensembles to brain-wide functional dynamics.A new tapered fiber optic design has recently been proposed to address this, enabling the illumination of relatively large volumes with comparatively homogeneous light delivery3 (Fig. 1). Here we describe proof-of-concept experiments designed to perform network-scale manipulations of neural activity by combining tapered fiber-based optical stimulation and mouse fMRI.
Methods
To test the stimulation of different neuronal populations we used 3 groups of mice expressing channelrhodopsin-2 (ChR2) and one group of opsin-free controls (details in Fig. 2). Tapered fibers were implanted in the brain such that the insertion point was in the somatosensory cortex and the fiber tip was in the dorsal hippocampus. Immediately after implantation, blood oxygen level dependent (BOLD) images were acquired on a 7T scanner (Bruker) using an echo planar imaging gradient echo (EPI-GE) sequence with the following parameters: TE=14ms, TR=1.2s, flip angle=30o, NR=280, matrix size=115x100, slice number=15, resolution=2x2x6mm. All scans were acquired under 0.8% isoflurane.Optical stimulation was performed at frequencies of 20Hz (15ms pulses) or 1Hz (10ms pulses) and with power densities of 20 or 100mW/mm2. Resting state fMRI (BOLD scans without optical stimulation) was also acquired. BOLD images were reconstructed and preprocessed as previously described4. 20Hz-BOLD scans were processed to identify regions with significant stimulation response using a Fourier analysis paradigm1,5. Functional connectivity (FC) from rsfMRI or 1Hz-BOLD scans was quantified using seed-based correlations, where a seed was chosen in the somatosensory cortex. Fisher transformed Pearson correlation coefficient values were fit with a linear mixed effect model6,7 probing the effect of stimulation (rsfMRI vs. 1Hz-BOLD) while accounting for a linear signal drift over scanning time and including a subject-specific random intercept. Voxelwise FC analyses were corrected for multiple comparisons using the false discovery rate (FDR) method8.
Results and discussion
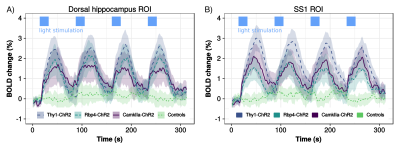
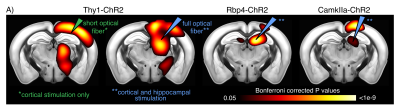
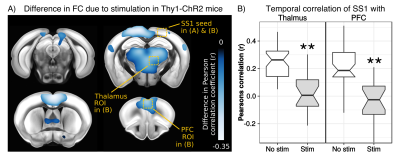
To test for possible heat-induced hemodynamic responses, we implanted control mice (which did not express ChR2) with tapered fibers and stimulated at 20Hz with increasing power densities. Contrary to previous observations with flat fibers2, we found that tapered fibers could be used to perform widespread illumination at power densities up to 100mW/mm2 without any observable changes in BOLD response (Fig. 3). In all the investigated experimental groups expressing ChR2, optogenetic stimulation at 20Hz with a single tapered fiber induced robust BOLD signal changes in both cortical and dorsal hippocampal areas irradiated (Fig. 4). A separate group of Thy1-ChR2 mice was implanted with a short probe illuminating only the cortex (Fig. 4, left). Importantly, the response pattern in these animals revealed cortical and unimodal thalamic activation, but not hippocampal engagement. This confirmed that the spatial extent of the observed cortico-hippocampal activation was the result of direct irradiation, and not a downstream feedforward activation.On top of spatial selectivity, optogenetics also has the benefit of tight temporal control, providing the opportunity to study the effect of neuronal oscillatory frequency on whole-brain functional network dynamics. To this end, we also performed low-frequency (1Hz) optical stimulation of both the SS1 and dorsal hippocampus in Thy1-ChR2 mice, in order to probe the dynamic rules governing patterns of synchronized activity in latero-cortical, hippocampal, and antero-posterior associative cortices4. When compared to stimulation free rsfMRI, we found that low frequency optogenetic stimulation led to a robust decoupling of the somatosensory cortex from thalamo-frontal components of the mouse default mode network (Fig. 5A). In particular, regions in the thalamus and prefrontal cortex exhibited significantly lower functional connectivity (Fig. 5B). This corroborates the idea of intrinsic competing dynamics between these functional systems4.
Conclusions
Taken together, these results pave the way to the use of tapered fiber opto-fMRI for the manipulation of large brain regions with precise temporal control. We propose the use of this platform as an attractive alternative to chemogenetics, when temporally precise activation or deconstruction of network-scale dynamics are required.Acknowledgements
This work was supported by the European Research Council (ERC – DISCONN; no. 802371 to A.G.)References
1. J. H. Lee et al., “Global and local fMRI signals driven by neurons defined optogenetically by type and wiring.,” Nature, vol. 465, no. 7299, pp. 788–92, Jun. 2010.
2. I. N. Christie et al., “FMRI response to blue light delivery in the naïve brain: Implications for combined optogenetic fMRI studies,” Neuroimage, vol. 66, pp. 634–641, 2013.
3. F. Pisanello et al., “Dynamic illumination of spatially restricted or large brain volumes via a single tapered optical fiber.,” Nat. Neurosci., vol. 20, no. 8, pp. 1180–1188, Aug. 2017.
4. D. Gutierrez-Barragan, M. A. Basson, S. Panzeri, and A. Gozzi, “Infraslow State Fluctuations Govern Spontaneous fMRI Network Dynamics,” Curr. Biol., vol. 29, no. 14, pp. 2295-2306.e5, Jul. 2019.
5. Z. Liang, G. D. R. Watson, K. D. Alloway, G. Lee, T. Neuberger, and N. Zhang, “Mapping the functional network of medial prefrontal cortex by combining optogenetics and fMRI in awake rats.,” Neuroimage, vol. 117, pp. 114–23, Aug. 2015.
6. D. Bates, M. Mächler, B. Bolker, and S. Walker, “Fitting Linear Mixed-Effects Models Using lme4,” J. Stat. Softw., vol. 67, no. 1, 2015.
7. A. Hrong-Tai Fai and P. L. Cornelius, “Approximate F-tests of multiple degree of freedom hypotheses in generalized least squares analyses of unbalanced split-plot experiments,” J. Stat. Comput. Simul., vol. 54, no. 4, pp. 363–378, Jun. 1996.
8. Y. Benjamini and Y. Hochberg, “Controlling the false discovery rate: a practical and powerful approach to multiple testing,” J. R. Stat. Soc. Ser. B, pp. 289–300, 1995.
Figures