3950
fMRI investigation of brain plasticity after unilateral cervical spinal cord injury
Basavaraju G Sanganahalli1, Jyothsna Chitturi2, Stella Elkabes3, Peter Herman1, Fahmeed Hyder1, and Sridhar S kannurpatti2
1Radiology and Biomedical Imaging, Yale University School of Medicine, New haven, CT, United States, 2Radiology, RUTGERS-New Jersey Medical School, Newark, NJ, United States, 3Department of Neurological Surgery, RUTGERS-New Jersey Medical School, Newark, NJ, United States
1Radiology and Biomedical Imaging, Yale University School of Medicine, New haven, CT, United States, 2Radiology, RUTGERS-New Jersey Medical School, Newark, NJ, United States, 3Department of Neurological Surgery, RUTGERS-New Jersey Medical School, Newark, NJ, United States
Synopsis
To assess brain plasticity after a unilateral incomplete cervical spinal cord injury (SCI), a complete neural axis assessment was performed using the adult rat model of cervical SCI at the C2/C3 level. Structural T1 and T2-spinal cord anatomical imaging and resting state BOLD-fMRI (9.4T) of the brain was performed 1 month after SCI in sham and SCI animals. The unilateral SCI induced lesion was discernable from anatomical images. Intrinsic brain activity assessed using functional connectivity density (FCD) mapping of global, short-range and long-range connectivity revealed a significant increase in FCD across SCI animals compared to sham.
Background
Neuroanatomical plasticity after a unilateral incomplete cervical spinal cord injury (SCI) at C3/C4 level comprises of sprouting of spared fibers (compensatory plasticity) and regrowth of severed fibers (regenerative plasticity)1. This leads to behavioral recovery of the fore and hindlimb motor functions due to reorganization of reticulospinal fibers sprouting above the spinal lesion and connecting to C3/C4 propriospinal neurons1. To assess brain plasticity after such a unilateral incomplete SCI, a complete neural axis assessment was performed using the adult rat model of cervical SCI at the C2/C3 level. Structural T1 and T2-spinal cord anatomical imaging and resting state Blood Oxygen Level Dependent (BOLD) functional Magnetic Resonance Imaging (MRI) of the brain was performed in sham and SCI animals. SCI was performed at the age of 2.5 months and imaged 1 month after SCI.Methods
All animal procedures were approved by the RUTGERS and YALE University IACUCs and in accordance with NIH guidelines. Female Sprague Dawley rats at 2.5 months age were randomly assigned to sham and SCI groups. SCI animals were anesthetized with Ketamine/Xylazine (80/10 mg/Kg, i.p) and laminectomized unilaterally on the left side between C2 and C3. A horizon impactor with a 2 mm tip diameter, 4 mm displacement and 150 Kdyne force was used to perform a mild to moderate impact injury on the exposed dura in 4 animals. Sham animals underwent laminectomy without the impact injury. Post SCI, muscle layers were sutured using absorbable suture and skin incision closed using staplers. Animals were provided 1 ml saline i.p to prevent dehydration and Buprenorphine SR analgesia (0.03 mg/Kg, subcutaneous) after surgery. Animals were monitored twice a day during the first 48 hours after SCI and daily thereafter. Structural and functional MRI was performed 1 month after SCI. MRI data were obtained on a modified 9.4 Tesla system with a Bruker spectrometer (Bruker, Karlsruhe, Germany) and custom-built 1H ellipsoidal surface coil (5 × 3 cm). Animals were anesthetized using dexmedetomidine anesthesia during imaging2, and body temperature was monitored throughout the procedure and maintained at 35-37ºC. Spinal cord anatomical reference images (T1 RARE (TR /TE = 4000/5.8 ms), T2 RARE (TR /TE = 4000/33 ms), four averages) were acquired in a 256× 256 matrix, for an effective in-plane resolution of 100 × 100 μm. Functional MRI Images were acquired over 12 contiguous coronal slices (thickness = 1 mm), covering the parenchyma between the olfactory bulb and cerebellum using single shot gradient echo planar imaging (GE-EPI). Functional images were acquired in a 64 × 32 matrix, for an effective in-plane resolution of 500 × 500 μm. For the resting-state paradigm, each scan lasted for 5 min (300 repetitions), repeated three times per animal3. All sham (n=4) and SCI (n=4) animal brain images were registered to the same template space of a typical sham animal. For resting-state functional connectivity analysis, EPI images were pre-processed by correcting for slice time and motion. Subsequently, images were linearly detrended, band-pass filtered (0.01–0.15 Hz), and spatially smoothed using a Gaussian filter (FWHM = 1.5 mm).Results
SCI led to severe immobility with both fore and hind limb debilitation up to 48 hours. Thereafter locomotion recovered gradually with complete hindlimb recovery at 1 month after SCI. While the contralesional forelimb recovered completely, ipsilesional forelimb usage continued to be significantly debilitated. Forepaw and hindpaw sensorimotor behavioral responses in sham and SCI groups are shown in Figure.1 and Figure.2.The unilateral SCI induced lesion was discernable from the T1 and T2-weighted anatomical images across all injured subjects (n=4), whereas no lesions were found across sham animals (n=4) (Figure. 3). Intrinsic brain activity assessed using functional connectivity density (FCD) mapping of global, short-range and long-range connectivity4 revealed a significant increase in FCD across SCI animals compared to sham (Figure.4). Furthermore, no interhemispheric FCD asymmetry was observed from the group analysis of sham and SCI animals.Discussion
In the hindlimb sensorimotor behavior recovered and ipsilesional forelimb debilitated animals after incomplete cervical SCI, the results indicate significant unilateral spine lesions accompanied by large scale brain reorganization leading to increased intrinsic brain activity. No interhemispheric asymmetry in intrinsic activity was observed despite behavioral asymmetry in forelimb utilization. Future forelimb and hindlimb stimulation-induced BOLD-fMRI studies may potentially reveal the altered functional representations in this model of incomplete SCI and whether they are related to the observed intrinsic brain activity changes.Acknowledgements
Funding from the New Jersey Commission for Brain Injury research CBIR12PIL028 (S.K.), CBIR15IRG010 (S.K.), R01 MH-067528 (F.H.), and P30 NS-052519 (F.H.) are acknowledged.References
- Filli L, Engmann AK, Zörner B, Weinmann O, Moraitis T, Gullo M, Kasper H, Schneider R, Schwab ME. "Bridging the gap: a reticulo-propriospinal detour bypassing an incomplete spinal cord injury." J Neurosci. 2014; 34(40):13399-13410.
- Shu C.Y, Sanganahalli BG, Coman D, Herman P, Hyder F. New horizons in neurometabolic and neurovascular coupling from calibrated fMRI. Progress in Brain Research.2016;225: 99–122.
- Parent M, Li Y, Santhakumar V, Hyder F, Sanganahalli BG, Kannurpatti SS. Alterations of parenchymal microstructure, neuronal connectivity and cerebrovascular resistance at Neurotrauma. 2019; 36(4):601-608
- Tomasi, D. and N. D. Volkow. Functional connectivity density mapping. Proc Natl Acad Sci U S A. 2010;107(21): 9885-9890.
Figures
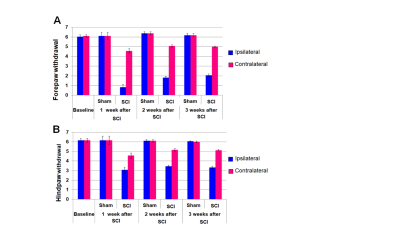
Figure
1. Forepaw (FSIMR) and hindpaw (HSIMR) sensorimotor
behavioral responses in sham (n=4) and SCI (n=5) groups. A. Forepaw
withdrawal responses to a dorsal tactile stimulus. B. Hindpaw withdrawal responses to a dorsal
tactile stimulus. Error bars represent
SEM.
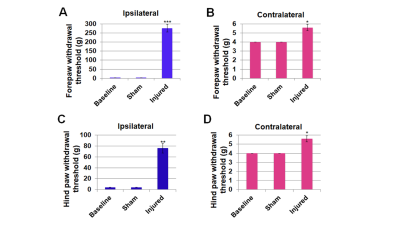
Figure
2. Sensory-evoked limb responses assessed by Von Frey
filament test at baseline before sham (n=4) or SCI (n=5) surgery and 3-weeks
after SCI. A. Ipsilateral forepaw had the highest sensory debilitation
(300g), C. followed by ipsilateral hindpaw (78g). B, D.
Hindpaws both ipsilateral and contralateral were mildly debilitated
(5.5g). Significantly different;
***p<0.0001; **p<0.001; *p<0.01 compared to baseline; One-way repeated
measures ANOVA. Data represent mean±SEM.
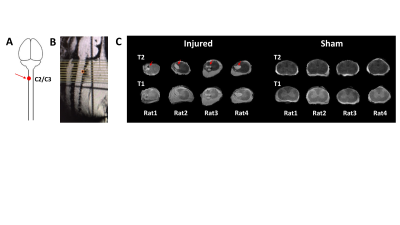
Figure 3. A: Schematic illustration of the adult rat models
of unilateral incomplete cervical spinal cord injury (SCI) at C2/C3 level used
in this study. Dorsal column on the left side was injured (red arrow) at the
C2/C3 spinal level. B: Sagittal view of invivo anatomical imaging of the spinal
cord showing the position of the imaging slices. C: T2 and T1 weighted spinal
cord anatomical images (coronal slices) form injured (red arrow indicates the
injured area) and sham rats
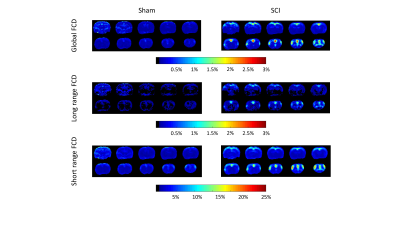
Figure 4. Average functional
connectivity density (FCD) maps of Sham and unilateral incomplete cervical
spinal cord injury (SCI) rats. The global FCD gives number of those voxels
which have larger than 0.7 Pearson’s correlation to a given voxel in the
percentage of all voxels. The short range FCD gives the percentage of those
voxels which have larger 0.7 Pearson’s correlation and are not farther than 3
mm from the given voxel. The long range FCD gives the percentage of those
voxels which are farther than 3 mm from the given voxel, but their correlation
is larger than 0.7.