3949
Orientation Selective Deep Brain Stimulation of Entorhinal Cortex and Medial Septal Nucleus in rats - functional MRI study at 9.4T.1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Department of Medicine, Surgery and Dentistry, Scuola Medica Salernitana, University of Salerno, Salerno, Italy, 3Department of Neurosurgery, University of Minnesota, Minneapolis, MN, United States, 4Department of Psychiatry, University of Minnesota, Minneapolis, MN, United States
Synopsis
Orientation selective deep brain stimulation (OS-DBS) is a flexible strategy to achieve selective stimulation of brain pathways. OS-DBS was used here in rats for stimulating the entorhinal cortex (EC) and the medial septal nucleus (MSN). Stimulating such areas induce neurogenesis in dental gyrus of the hippocampal formation, and can thus be regarded a potential therapy for Alzheimer’s disease (AD). Brain responses during OS-DBS were monitored by MB-SWIFT fMRI at 9.4T. Results demonstrate that different orientations of OS-DBS can modulate and selectively maximize activation of EC/MSN- hippocampal formation networks, and thus hold promise for maximizing neurogenesis and therapy efficacy for AD.
Introduction
Deep brain stimulation (DBS) has proved to be an effective tool for treating neurological and neuropsychiatric disorders1. To improve the spatial selectivity of neuromodulation with DBS, we recently introduced a novel orientation-selective strategy for DBS (OS-DBS)2. By using multiple contacts with independent current sources, the electric field can be oriented along the desired orientation in space to induce the activation of a selected pathway, reaching maximal efficiency when electric field gradients are parallel to the axons3. In this work, the OS-DBS strategy was used for stimulating the entorhinal cortex (EC) and the medial septal nucleus (MSN). Such brain areas play an important role in memory-related and spatial computation functions and are critical in the therapy of Alzheimer’s disease (AD) using DBS. The OS-DBS could offer a new degree of optimization of DBS treatment for AD. To evaluate the efficacy of stimulation, brain responses were monitored by means of fMRI in rats. Our results show that by changing the orientation of the electrical field, OS-DBS can significantly modulate the activity of the hippocampus, amygdala, hypothalamus, prelimbic cortex and other cortical networks connected to the EC and MSN.Materials and Methods
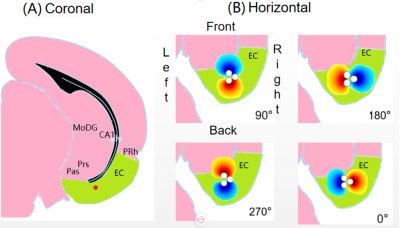
All surgical and experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Minnesota. A total of 19 Sprague-Dawley rats were used. After the electrode implantation, MRI scans were conducted with a 9.4-T 31-cm horizontal-bore magnet under urethane (1/160 ml per rat’s weight in g, concentration 6g urethane/25ml saline (5%)). The stimulation paradigm consisted of 60 s of rest and 18 s of stimulation, repeated three times and ending in a rest period. The placement of the electrode and the response of the brain to stimulus were confirmed by driving the tripolar electrode in monopolar configuration (i.e, biphasic, 200 µs per phase, 1–2 mA divided between the three contacts, 20 Hz for EC and 130 Hz for MSN). For OS-DBS the relative current amplitudes of the three channels were chosen based on sinusoidal functions with phase offsets of 120°: I1=Icos(f) I2=Icos(f+120°) I3=Icos(f-120°). MB-SWIFT images were reconstructed by gridding with three iterations FISTA algorithm4. The resulting data were analyzed in SPM8 (www.fil.ion.ucl.ac.uk/spm). To quantify the differences of the fMRI responses to different OS-DBS angles, aggregates of MB-SWIFT fMRI data were then obtained in anatomically defined regions of interest (ROIs) drawn manually with Aedes (aedes.uef.fi) based on a rat brain atlas5.Results
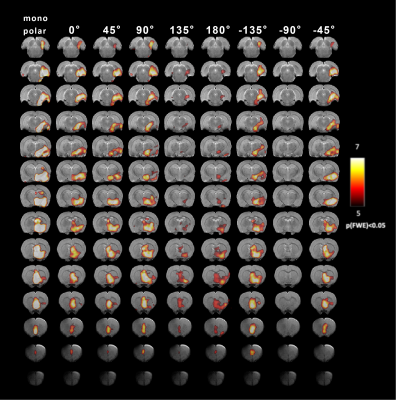
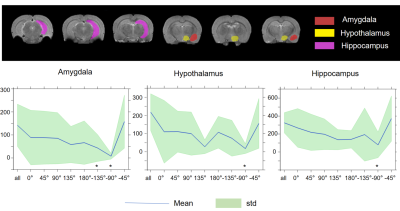
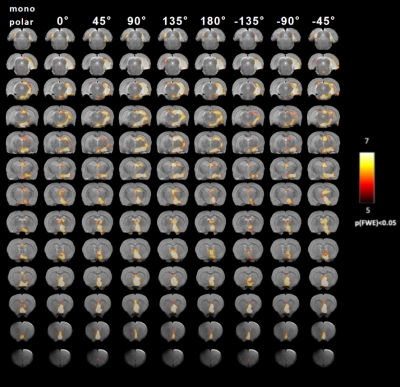
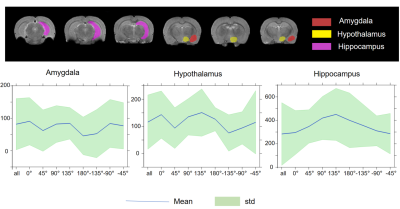
In nine out of twelve animals, the implantations in the EC were confirmed using histological analysis, in three out of twelve rats the precision of implantation was inspected by T2-weighted anatomical images. All twelve data sets were included in the analysis. For MSN, seven animals with implantations in MSN were inspected by T2-weighted anatomical images and were included in the analysis. The results of main effect of the stimulation of the EC are shown on the activation maps of group analysis in Fig.2. Broad spread of activation was observed as a result of OS-DBS at 0°, -135°, -45°, and reduced activity was observed at the angles -90°, 135°, and 180°. The weakest activation was detected with stimulation angles -90°. Overall, OS-DBS of the EC induced modulation of related networks depending on the orientation of the stimulus including the hippocampus, amygdala, prelimbic cortex, infralimbic cortex (IL) ( Fig.3). OS-DBS of the MSN leads to a strong fMRI response in the hippocampus, with the strongest activation achieved around 135°. Smaller effects of the orientation of stimulation were observed in the amygdala and hypothalamus (Figs 4&5).Discussion
In this study, we demonstrate that OS-DBS can be used to modulate the activity of the EC/MSN- hippocampal formation networks. Significant dependence of the activation on specific angles during OS-DBS suggested that OS-DBS of EC and MSN can allow recruiting specific neuron populations, and can selectively maximize stimulation of the hippocampus and the other areas connected to EC and MSN. Further work will focus on evaluating OS-DBS of the EC and MSN using leads with higher channel numbers and with the capability of achieving orientation selectivity in 3D space rather than in plane only. In addition, future work will verify whether the orientation of stimulations which maximize hippocampus activation in acute OS-DBS experiments also leads to stronger neurogenesis effects after chronic OS-DBS.Conclusion
Our results demonstrate that OS-DBS of the EC can modulate brain network activity in the hippocampus. OS-DBS of the MSN also demonstrated a high degree of orientation selectivity of activation of the hippocampus. Future investigation will focus on evaluating neurogenesis induced by OS-DBS of MSN and EC in the dental gyrus of the hippocampal formation.Acknowledgements
This work was supported by the National Institutes of Health supplement 3U01NS103569-03S1 to the U01-NS103569-01 grant, the Center for Magnetic Resonance Research NIH core grant P41EB027061, the EU H2020 Marie Skłodowska RISE project #691110 (MICROBRADAM).References
1. Edwards, C.A., et al., Neurostimulation Devices for the Treatment of Neurologic Disorders. Mayo Clin Proc, 2017. 92(9): p. 1427-1444.
2. Lehto, L.J., et al., Orientation selective deep brain stimulation. J Neural Eng, 2017. 14(1): p. 016016.
3. Rattay, F., Analysis of models for extracellular fiber stimulation. IEEE Trans Biomed Eng, 1989. 36(7): p. 676-82.
4. Beck, A. and M. Teboulle, Fast gradient-based algorithms for constrained total variation image denoising and deblurring problems. IEEE Trans Image Process, 2009. 18(11): p. 2419-34.
5. Swanson, L.W., Brain maps 4.0-Structure of the rat brain: An open access atlas with global nervous system nomenclature ontology and flatmaps. J Comp Neurol, 2018. 526(6): p. 935-943.
Figures