3944
Characterizing the morphology and resting-state functional connectivity in chronic specific and nonspecific low back pain1Second Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
Synopsis
It is important to determine and distinguish the mechanisms underlying different types of pain because different drug targets are useful in pain of different origins. Specific low back pain (SLBP) with a recognizable pathology and nonspecific low back pain (NSLBP) are different pain conditions. The amygdala has been linked with the pathophysiology of chronic LBP. However, it is not known whether the amygdala is differentially affected in both conditions. Our study suggested that the amygdala morphology, resting-state functional connectivity and effective connectivity are differentially affected in subjects with SLBP and NSLBP, indicating different brain mechanisms in SLBP and NSLBP.
Main Body
INTRODUCTIONLow back pain (LBP) is the leading cause of disability worldwide and is a difficult condition to effectively treat due to various etiologies [1]. Of all LBP, only a small portion of people have a specific pathological cause, the majority of LBP patients (almost up to 90%) are not possible to identify a specific nociceptive cause, which are classified as nonspecific LBP (NSLBP) [2]. It is important to determine and distinguish the mechanisms that account for different types of pain, because different drug targets may be useful in pain of different origins. The amygdala has been linked with the pathophysiology of chronic LBP by presence of smaller volume [3, 4], increased amplitude of low-frequency fluctuation [5], exaggerated and abnormal connectivity [6, 7]. However, most earlier studies targeting amygdala role in LBP didn’t differentiate NSLBP and SLBP. Little attention has been paid to that whether the amygdala is differentially affected in specific LBP (SLBP) with a clear cause and NSLBP.
METHODS
Thirty-three subjects with NSLBP, 33 with SLBP, and 33 healthy controls (HCs) were examined. The volume and surface morphology of amygdala were determined by FSL-FIRST software. The resting-state functional connectivity (rsFC) of amygdala was explored using seed-based connectivity in CONN toolbox. The effective connectivity of the amygdala were measured by dynamic causal modelling.
RESULTS
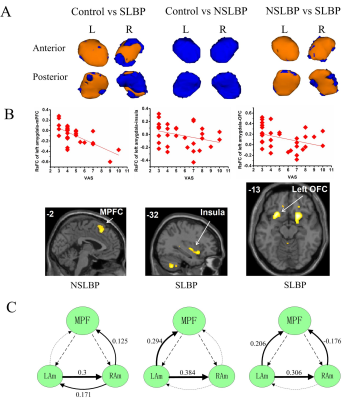
Subjects with SLBP had reduced normalized volumes of the bilateral amygdala when compared with HCs; there was no significant volume difference between NSLBP and HCs (Figure 1A). Connectivity analysis revealed increased rsFCs of the right amygdala-parieto-occipital region in SLBP and increased rsFC of the left amygdala-medial prefrontal cortex (mPFC) in NSLBP as compared to HCs. The amygdala rsFCs in NSLBP (left amygdala-mPFC) and SLBP (left amygdala-OFC, left amygdala-insula) were negatively correlated with pain intensities (Figure 1B). NSLBP decreased effective connectivity from the mPFC to the left amygdala, and SLBP decreased effective connectivity from mPFC to the right amygdala when compared with HCs (Figure 1C) and both are negatively associated with pain intensities.
DISCUSSION
These findings, together with the negative correlations between pain intensities and amygdala rsFCs in both the NSLBP and SLBP groups, might reflect a divergent affective/cognitive processing via amygdala and pain-related maladaption of the amygdala in these two LBP cohorts. A potential limitation of this study is that this is a cross-sectional design while the progression of LBP is divergent. A longitudinal study would be helpful to understand the causal relationships of the amygdala alterations and pathogenesis of NSLBP and SLBP.
CONCLUSION
In conclusion, we found that SLBP, but not NSLBP, is associated with amygdala morphological changes although both NSLBP and SLBP are associated with altered amygdala rsFC and effective connectivity. These findings may imply the divergent brain pathogenesis underlying SLBP and NSLBP. Illucidating amygdala-cortical circuits underlying LBP patient with different etiologies may shed light on the brain pathophysiology of LBP, facilitate the development of individualized pain managements and improve the care of chronic pain.
Acknowledgements
This research was supported by grants from China Scholarship Council (201806285075), the National Natural Science Foundation of China (81501455), and the Natural Science Foundation of Shaanxi Province (2018SF-135).References
[1] Hartvigsen, J, Hancock, MJ, Kongsted, A, et al. What low back pain is and why we need to pay attention[J]. The Lancet, 2018. 391(10137): 2356-2367.
[2] Maher, C, Underwood, M, Buchbinder, R. Non-specific low back pain[J]. Lancet, 2017. 389(10070): 736-747.
[3] Mao, CP and Yang, HJ. Smaller Amygdala Volumes in Patients With Chronic Low Back Pain Compared With Healthy Control Individuals[J]. J Pain, 2015. 16(12): 1366-1376.
[4] Lin, JC, Chu, LF, Stringer, EA, et al. One Month of Oral Morphine Decreases Gray Matter Volume in the Right Amygdala of Individuals with Low Back Pain: Confirmation of Previously Reported Magnetic Resonance Imaging Results[J]. Pain Med, 2016. 17(8): 1497-504.
[5] Zhang, B, Jung, M, Tu, Y, et al. Identifying brain regions associated with the neuropathology of chronic low back pain: a resting-state amplitude of low-frequency fluctuation study[J]. Br J Anaesth, 2019. S0007-0912(19): 30143-30146.
[6] Jiang, Y, Oathes, D, Hush, J, et al. Perturbed connectivity of the amygdala and its subregions with the central executive and default mode networks in chronic pain[J]. Pain, 2016. 157(9): 1970-8.
[7] Meier, ML, Stampfli, P, Humphreys, BK, et al. The impact of pain-related fear on neural pathways of pain modulation in chronic low back pain[J]. Pain Rep, 2017. 2(3): e601.
[8] Apkarian, AV, Sosa, Y, Sonty, S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density[J]. J Neurosci, 2004. 24(46): 10410-5.
[9] Gustin, SM, Peck, CC, Wilcox, SL, et al. Different pain, different brain: thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes[J]. J Neurosci, 2011. 31(16): 5956-64.
Figures