3941
Understanding the functional connectivity of neural engagement of motor imagery and task through EEG engagement index informed fMRI1NMR Research centre, Institute of Nuclear Medicine and Allied Sciences, DRDO, New Delhi, India, 2Maharaja Surajmal Institute of Technology, New Delhi, India, 3Banasthali Vidyapith, Rajasthan, India
Synopsis
Understanding neural engagement of Motor imagery facilitates development of various Brain-Computer Interface systems for rehabilitation purposes effectively. This study brings more insights on the neural underpinnings and associated functional connectivity of basal-ganglia, temporal and frontal-parietal regions during both Motor Imagery (MI) and Motor Action (MA) gripping tasks of randomised left, right and both hands movement with fifteen right-handed volunteers, using EEG-engagement index informed fMRI and functional connectivity approach. Functional connectivity analysis of the neural correlates reveal statistically elevated engagement of basal-ganglia, superior temporal gyrus and frontal-parietal regions in imagery, left handed imagery/action and both hand gripping tasks respectively.
Introduction
Understanding the functional connectivity of neural task engagement associated with Motor Imagery (MI) as compared with Motor Action (MA) is one of the core interests in Brain Computer Interface (BCI) field as it enables us to design better rehabilitation solution1.One of the challenges2 in understanding the neural engagement of the MI is that recruitment of differential sensory processing and attention resources based on types of imagery and skill level of the individual (e.g., novice or trained). Hence, to facilitate a better understanding of the engagement of each neural process, an EEG-engagement index informed fMRI analysis is carried out for Motor imagery over Motor action in this study. Further, more insight is brought to the analysis of functional connectivity using graph theory to understand the global and local efficiency measures of the observed regions of interest.Methods
Fifteen healthy right-handed volunteers (10 males and 5 females; Mean age: 22.78 years) participated in a simultaneous EEG - fMRI investigation using 3T MRI (Siemens) and 32 Channel MR Compatible EEG (Brain Product) system. Each volunteer was subjected to randomized motor imagery and motor action tasks for left, right and both hands grip / release operations. Each grip and release MI/MA tasks were given 4s time independently and followed by 4 seconds fixation block (Figure 1). Subsequently, the simultaneously acquired EEG data is pre-processed for MR related artifacts, eye blink, motion artifact removal using BrainVision Analyzer. EEG data was downsampled to 250 Hz and bandpass filtered between 1 Hz and 50 Hz using an FIR filter and finally re-referenced without ECG data channel. The EEG data was then wavelet transformed to estimate 0-4, 4-8, 8-12, 12-30 and 30-50 Hz wavelet bands to capture relative delta, theta, alpha, beta, and gamma frequency values for each MI and MA blocks from Fz-POz EEG channels. Further, in order to approximate the demands for sensory processing and attention resources, the EEG engagement index was computed as the ratio of relative beta with the sum of relative alpha and relative theta. Using an averaged band wave from all sensors, (Beta/(Alpha + Theta)) provided the best solution as it has lower overhead for implementation along with the benefit of mitigating noise from individual sensor locations3. For processing functional MRI images, SPM12 toolbox of MATLAB was used for slice time correction, realignment, and reslicing, segmentation, normalization, smoothening and co-registration. First-level analysis was carried out with the estimated engagement index as a parametric modulator for each motor imagery and task conditions. Furthermore, the second-level analysis was done using robust regression6 that eliminates the possible outliers that may lead to incorrect inferences. Finally, the results of the second-level analysis were subjected to one way t-statistic method and significant activations were analyzed for contrasts MAEI-MIEI (left hand, right hand, and both hands) at family-wise error (FWE) corrected p < .001 significance. The result was then evaluated using the Harvard-Oxford cortical and subcortical atlases and neural correlates corresponding to engagement during MA-MI (left hand, right hand, and both hands) were analyzed. Subsequently, these neural correlates were used as Regions Of Interest (ROI) for functional connectivity analysis using CONN toolbox4 in MATLAB. The functional connectivity analysis was carried out for both MI and MAtask of left, right and both hands. The Global efficiency (GE) and Local efficiency (LE)parameters were calculated using ROI-ROI analysis and the results were then compared and analyzed.Results
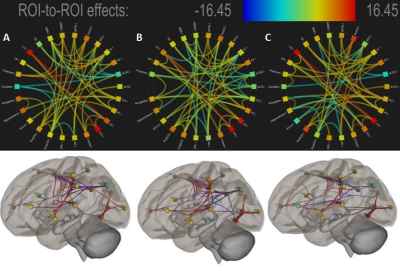
The Mean Engagement-Index assessed for motor imagery (EI-Left_hand=0.508±0.122, EI-Right_hand= 0.528±0.13, EI-Both_hand=0.535±0.16) and motor action (EI-Left_hand=0.527±0.166, EI-Right_hand=0.529±0.166, EI-Both_hand=0.539±0.17)tasks has revealed statistically significant differences. Further, neural underpinning of MAEI-MIEI as assessed by EEG based engagement index informed fMRI analysis is shown in Figure 2. Finally, the functional connectivity analyses of the neural underpinnings of Engagement Index are shown in Figure 3 and 4. Both neural underpinning and associated functional connectivity revealed strong engagement of basal ganglia regions during imagery (EI-left_hand:GE=7.74,LE=7.26[Amygdala_right]; EI-Right_hand:GE=9.59,LE=3.65[Caudate_right]; EI-Both_Hand: GE=11.2,LE=5.48 [Putamen_left]) compared to MA (EI-Left_hand:GE=7.83,LE=3.74[Amygdala_right]; EI-Right_hand: GE=0.0,LE = 0.0[Caudate_right]; EI-Both_hands: GE=8.39,LE=3.59[Putamen_left]). In addition, Superior temporal gyrus is strongly engaged during both left imagery (GE=13.07,LE=5.06) and action gripping (GE=24.55,LE=3.79)as compared to right hand gripping(MI:GE=10.48,LE=4.59; MA:GE=11.81,LE=5.18) and both hand gripping (MI:GE=20.22,LE=4.45; MA:GE=8.85,LE=4.40). Further, engagement of frontal-parietal regions such as Superior Parietal Lobule (SPL) and Superior Frontal Gyrus (SFG)was observed to be higher during both hand griping (GE=18.24,LE=2.72[SPL]; GE=17.81,LE=7.28[SFG]).Discussion and Conclusion
In the study, the neural underpinning and associated functional connectivity of motor imagery as compared with motor action has clearly brought out the following understandings. At first, the engagement of basal ganglia regions7,8 such as caudate, putamen and amygdala are observed primarily during motor imagery as compared to motor action. The study has also observed elevated involvement of superior temporal9 regions during left motor imagery and motor action. As most of the volunteers were right-handed, it clearly establishes the role of memory retrieval during the left-handed imagery and action. Further, the study has clearly established the elevated involvement of fronto-parietal regions10 during motor imagery gripping tasks as compared to the motor actions. Thus, this study brings better understandings of engagement of basal-ganglia, temporal and frontal-parietal regions during motor imagery/action through EEG engagement index informed fMRI analysis and associated the functional connectivity approach.Acknowledgements
No acknowledgement found.References
- Xu, L., et al. "Motor execution and motor imagery: a comparison of functional connectivity patterns based on graph theory." Neuroscience 261 (2014): 184-194.
- Tacchino, Giulia, et al. "EEG Analysis during active and assisted repetitive movements: evidence for differences in neural engagement." IEEE Transactions on Neural Systems and Rehabilitation Engineering 25.6 (2016): 761-771.
- McMahan, Timothy, Ian Parberry, and Thomas D. Parsons. "Evaluating player task engagement and arousal using electroencephalography." Procedia Manufacturing 3 (2015): 2303-2310.
- Whitfield-Gabrieli, Susan, and Alfonso Nieto-Castanon. "Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks." Brain connectivity 2.3 (2012): 125-141.
- Munzert, Jörn, Britta Lorey, and Karen Zentgraf. "Cognitive motor processes: the role of motor imagery in the study of motor representations." Brain research reviews 60.2 (2009): 306-326.
- Wager, T. D., Keller, M. C., Lacey, S. C., &Jonides, J. (2005). Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage, 26(1), 99-113.
- Doyon, Julien, et al. "Contributions of the basal ganglia and functionally related brain structures to motor learning." Behavioural brain research 199.1 (2009): 61-75.
- McInnes, Kerry, Christopher Friesen, and Shaun Boe. "Specific brain lesions impair explicit motor imagery ability: a systematic review of the evidence." Archives of physical medicine and rehabilitation 97.3 (2016): 478-489.
- Oostra, Kristine M., et al. "Damage to fronto-parietal networks impairs motor imagery ability after stroke: a voxel-based lesion symptom mapping study." Frontiers in behavioral neuroscience 10 (2016): 5.
Figures