3937
Reconstructing the BOLD-fMRI signal at the facial expression processing network from simultaneous EEG-derived predictors1CIBIT, Coimbra Institute for Biomedical Imaging and Translational Research, Faculty of Medicine, University of Coimbra, COIMBRA, Portugal, 2CIBIT, University of Coimbra, Coimbra, Portugal, 3CISUC, University of Coimbra, Coimbra, Portugal
Synopsis
fMRI is the neuroimage modality of choice when considering localized neurofeedback applications. However, the high costs and inflexibility of MRI setups limit their widespread application, motivating their transfer to EEG setups by reconstructing the BOLD-fMRI signal at the target regions using EEG only. Here, we systematically investigated the extent at which the BOLD-fMRI signal at the facial expressions processing network could be reconstructed from simultaneously recorded EEG signals. Features from both scalp and source spaces were extracted and used as predictors in a regression problem using random forests. We improved the accuracy of the state-of-the-art method from 20% to 53%.
Introduction
fMRI-based neurofeedback (NF) interventions represent the method of choice for the neuromodulation of localized brain areas1,2. Because of their economical and logistical constraints, transferring these interventions to EEG setups would promote their widespread application, due to their low cost and portability. This can be accomplished by reconstructing the BOLD-fMRI signal measured at the brain regions and/or networks to be targeted by the NF using only EEG signals. Despite its academic and clinical interest, such methodological strategy has been poorly explored so far. To the best of our knowledge, only Meir-Hasson and colleagues3 have attempted to reconstruct the BOLD-fMRI signal recorded at a specific region (the amygdala) from scalp EEG signals based on a ridge regression of power activity from different frequency bands and several time delays (the EEG Finger-Print method – EFP).To better understand the extent at which the BOLD-fMRI signal from a specific brain region or network can be accurately reconstructed from the EEG alone in the context of transferring NF interventions from fMRI into EEG, we systematically explored several EEG features extracted from both the scalp and source spaces as potential predictors of the BOLD signal recorded from the facial expression processing network (FEPN) during a NF task.
Methods
Data acquisition and pre-processing: Ten healthy subjects performed a simultaneous EEG-fMRI NF session on a 3T MRI system (Siemens) using an MR-compatible 64-channel EEG system (NeuroScan). BOLD-fMRI (2D-EPI, TR/TE=2000/30 ms) was acquired concurrently with EEG during four runs: a functional localizer and three neurofeedback runs4. EEG data were corrected for the MR-induced artefacts5,6 and band-pass filtered (1-45 Hz); fMRI data were subjected to advanced pre-processing steps7.fMRI data analysis: The FEPN was mapped using a GLM with boxcar functions convolved with a canonical HRF modelling each condition presented during the functional localizer. Voxels exhibiting significant signal changes when contrasting the facial expression conditions with the neutral and motion conditions were identified (voxel Z > 2.7, cluster p < 0.007). The BOLD signals within the FEPN were then averaged for each run and used as the ground truth signals to be reconstructed.
EEG data analysis: For each subject, we extracted seven EEG features from seven frequency bands of interest (theta, alpha, beta, low-beta, high-beta, gamma and broadband) from non-overlapping scalp EEG segments of 2 seconds (matching the TR of the fMRI data) from a subset of ten electrodes (five at each hemisphere) selected based on their proximity to the FEPN. For the source-based models, the EEG features were then mapped to the source space using continuous EEG source imaging8 (processing steps according to9), and their source time courses extracted from either the FEPN (ROI), or from 90 non-overlapping brain regions parceled according to the Automated Automatic Labeling (AAL) atlas.
BOLD reconstruction approach: Seven EEG features were considered to build the proposed pool of features (the FeatPool model), three from the time-frequency domain (power, frequency peak and the Teager energy) and the remaining four from the nonlinear domain (correlation dimension, the Lyapunov exponent, sample and approximate entropies), the latter to exploit those recognized characteristics of the EEG10–12. All features were estimated for each frequency band and for each 2-second EEG segment.
Dealing with the haemodynamic delay: Two methods were considered to deal with the time lag between EEG and the BOLD signal: the use of several time delays applied to the EEG predictors (as used by the EFP); and the convolution of the EEG predictors with a pool of haemodynamic response functions (HRF) peaking at different latencies.
Models analysed: For baseline we considered the state-of-the-art EFP model, which considers different delays. We compared it with the multiple HRF convolution approach of the power (iEFPscalp) and using all the features (FeatPoolscalp). We also analysed both models from the source space (iEFPsource and FeatPoolsource), as well as the direct mapping of the EEG broadband signal (EEGsource; Fig. 1). The models tested were compared in terms of their reconstruction accuracy (rACC), defined as the Pearson correlation between the measured and reconstructed BOLD signals.
Results
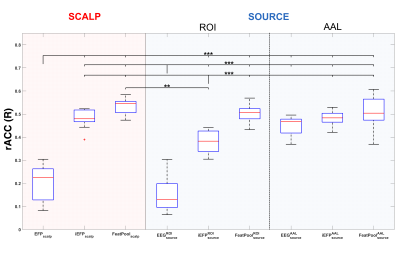
Comparing with the EFP model, only the EEGsourceROI model did not surpass its rACC (Fig. 2); in contrast, all the BOLD reconstructions from the other proposed models obtained statistically significantly higher rACC values. At the scalp level, the FeatPoolscalp outperformed the iEFPscalp model, showing that the pool of features can capture additional task-specific brain processes that are not fully identified using only the EEG power. At the source level, the same pattern was observed, with the FeatPoolsource exhibiting better results than the iEFPsource (both for ROI and AAL versions). As expected, using the information from several brain regions parceled according to the AAL, rather than the FEPN alone, yielded higher rACC values.Conclusion
We were able to reconstruct the BOLD signal measured at the FEPN more accurately by exploring nonlinear EEG features and convolving them with multiple HRF functions peaking at different latencies, which were able to account for the variability the hemodynamic delay of the BOLD signal. Our results may positively impact the transfer of fMRI-based NF interventions to EEG setups and their dissemination and efficacy in modulating the activity of the desired brain areas.Acknowledgements
Supported by The European Commission, under the Health Cooperation Work Programme of the 7th Framework Programme, with the Grant Agreement BRAINTRAIN—Taking imaging into the therapeutic domain: Self-regulation of brain systems for mental disorders [FP7-HEALTH- 2013-INNOVATION-1–602186 20, 2013]; FLAD Life Sciences, 2016; FTC - Portuguese national funding agency for science, research, and technology UID/4539/2013 – COMPETE, POCI-01-0145-FEDER-007440, PAC MEDPERSYST POCI-01-0145-FEDER-016428, SFRH/BD/77044/2011.
References
1. Fovet, T., Jardri, R. & Linden, D. Current Issues in the Use of fMRI-Based Neurofeedback to Relieve Psychiatric Symptoms. Curr. Pharm. Des. 21, 3384–3394 (2015).
2. Sitaram, R. et al. Closed-loop brain training: the science of neurofeedback. Nat. Rev. Neurosci. 18, 86–100 (2016).
3. Meir-Hasson, Y., Kinreich, S., Podlipsky, I., Hendler, T. & Intrator, N. An EEG Finger-Print of fMRI deep regional activation. Neuroimage 102, 128–141 (2014).
4. Direito, B. et al. Targeting dynamic facial processing mechanisms in superior temporal sulcus using a novel fMRI neurofeedback target. Neuroscience 406, 97–108 (2019).
5. Allen, P. J., Josephs, O. & Turner, R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage 12, 230–239 (2000).
6. Abreu, R. et al. Ballistocardiogram artifact correction taking into account physiological signal preservation in simultaneous EEG-fMRI. Neuroimage 135, 45–63 (2016).
7. Abreu, R., Nunes, S., Leal, A. & Figueiredo, P. Physiological noise correction using ECG-derived respiratory signals for enhanced mapping of spontaneous neuronal activity with simultaneous EEG-fMRI. Neuroimage 154, 115–127 (2017).
8. Simões, M. et al. A novel biomarker of compensatory recruitment of face emotional imagery networks in autism spectrum disorder. Front. Neurosci. 12, 1–15 (2018).
9. Bosl, W. J., Loddenkemper, T. & Nelson, C. A. Nonlinear EEG biomarker profiles for autism and absence epilepsy. Neuropsychiatr. Electrophysiol. 3, 1 (2017).
10. Bosl, W. J., Tager-Flusberg, H. & Nelson, C. A. EEG Analytics for Early Detection of Autism Spectrum Disorder: A data-driven approach. Sci. Rep. 8, 1–20 (2018).
11. Smith, S. M. et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 106, 13040–13045 (2009).
Figures