3936
Driving with distraction: brain activity and oculomotor behaviour using fMRI and eye-tracking1Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 3St. Michael's Hospital, Toronto, ON, Canada
Synopsis
This study sheds further light on the neural correlates of driving behaviour and distracted driving, an important road-safety issue. Functional MRI and simultaneous eye-tracking measurements are performed during simulated driving tasks with and without auditory distraction. Initial results are consistent with previously published fMRI findings, showing changes in the occipital lobes, temporal lobes and frontal regions that are associated with increasing cognitive demand and distraction. These observations, and their interpretation, are consistent with reductions in the gaze field-of-view and increases in pupil diameter that reflect how the brain deals with cognitive challenges during realistic driving scenarios.
Introduction
Driving is an essential daily activity that requires many complex cognitive abilities such as attention, working memory and decision-making. Studying the brain activity that supports driving behaviour is important as part of developing strategies for improved road safety. Previous functional magnetic resonance imaging (fMRI) studies have shown that multiple brain regions support simulated driving activities such as maintenance of speed, and performing turns with and without auditory distractions1–5. In addition, eye-tracking metrics (e.g. gaze, pupil diameter) have provided indices of attention and cognitive effort that show associations with increasing cognitive demand across various driving and mental tasks6–10. At present, no studies have investigated oculomotor behavioural changes combined with fMRI to assess brain activity in simulated driving. Therefore, the present study uses eye-tracking to further support and strengthen the interpretation of the associated fMRI brain activation patterns.Methods
The study involves fMRI of 20 healthy experienced drivers (ages 20-30) performing driving tasks inside a 3T MRI system (Prisma, Siemens) using STISIM Drive software (Systems Technology, Inc.) to control a custom fMRI-compatible driving simulator11; and simultaneous high-speed (1000 Hz) eye-tracking using a binocular system with a 50 mm lens (EyeLink 1000 Plus, SR Research Ltd.). Prior to fMRI, participants undergo one hour in a mock fMRI system to practice simulated driving. The driving tasks include straight driving, turning at intersections without (“Left Turn”) and with oncoming traffic (“Left Turn + Traffic”), and driving while performing audio tasks to simulate distracted driving (“Straight + Audio” and “Left Turn + Traffic + Audio”). Anatomical imaging is performed using 3D MPRAGE (TR=1.8 s, TE=2.21 ms, FA=10º, FOV=256x256 mm2, 176 slices, voxel size=1.0x1.0x1.0mm3). Functional imaging is performed using T2*-weighted EPI (TR=1.75 s, TE=30 ms, FA=40º, FOV=256x256 mm2, 60 slices, voxel size=2.5x2.5x2.5 mm3 in 377 frames). The fMRI data are preprocessed using AFNI freeware12 to perform motion correction, slice time correction, and spatial smoothing using a 5 mm FWHM Gaussian kernel, then normalized by the mean of each voxel. Brain activity is estimated using a general linear model with straight driving as the baseline condition, convolving a stimulus-timing file with a variable shape hemodynamic response function and nuisance regressors (including 6 head motion parameters), then averaged in Talairach atlas space13. The t-statistic maps are thresholded using a false discovery rate of q = 0.05 to correct for multiple statistical comparisons.Results
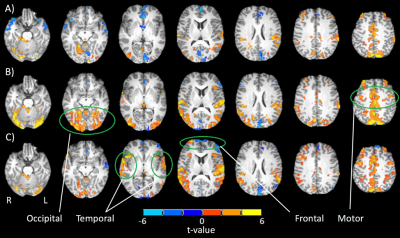
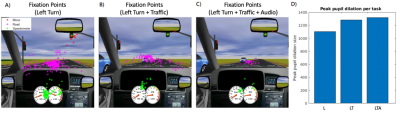
Initial t-statistic brain activation maps from a representative participant (24 years old) show left turns generating slight activation in occipital, cerebellar and motor areas (Fig. 1A), while left turns with oncoming traffic show greater extent and amplitude in these regions (Fig. 1B). In the case of distraction, adding auditory tasks to left turns with oncoming traffic introduced additional bilateral activations in the temporal-parietal and frontal regions (Fig. 1C), and an accompanying decrease in activation of visual, cerebellar and motor areas compared to the latter case when the task was performed without distraction. Fig. 2A-C shows gaze tracking on the driving simulation display screen, indicating that the participant alternated between studying the rear-view mirror, road and speedometer throughout, with their gaze field of view decreasing with increasing cognitive demand across the three tasks. Pupil dilation increases with increasing cognitive demand (Fig. 2D).Discussion
These brain activation results are consistent with previous findings and interpretation4. Visual, motor and cerebellar areas are predominantly and increasingly recruited when driving manoevres become more challenging, as expected for efficient neural processing of acquired complex skills. When distraction occurs, however (in this case, a simultaneous auditory task) frontal and temporal regions are recruited to undertake executive functions (e.g. plan, monitor performance, allocate attention) and additional task processing, respectively9,14. This additional recruitment is associated with decreased activation especially of visual and cerebellar regions, suggesting a re-allocation of resources to meet secondary processing needs. Supporting this interpretation, gaze maps representing patterns of visual exploration are seen to narrow as the tasks became more demanding, especially in the distracted driving task as the visual system was throttled to accommodate auditory task demands. Pupil diameter, known to index cognitive effort and arousal, also shows expected increases as the tasks became more challenging.Conclusions
A unique multi-measurement approach has been developed that combines eye-tracking with fMRI to measure brain and oculomotor behaviour during simulated driving across varying levels of complexity and distraction. The initial findings from this work, supported by gaze and pupil dilation data, further the understanding of how functional brain networks change under varied driving conditions, with recruitment of additional neural resources as task complexity increases and re-allocation of resources when there are competing task demands. Future work will investigate the relationships between regions of brain activation and changes in gaze and pupil dilation when driving, for the full cohort of participants.Acknowledgements
Support for this research is provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Ontario Graduate Scholarship (OGS).References
1. Calhoun, V. D. et al. Different activation dynamics in multiple neural systems during simulated driving. Hum. Brain Mapp. 16, 158–167 (2002).
2. Hyung-Sik, K. et al. Cerebral activation and lateralization due to the cognition of a various driving speed difference: An fMRI study. Biomed. Mater. Eng. 1133–1139 (2014) doi:10.3233/BME-130913.
3. Just, M. A., Keller, T. A. & Cynkar, J. A decrease in brain activation associated with driving when listening to someone speak. Brain Res. 1205, 70–80 (2008).
4. Schweizer, T. A. et al. Brain activity during driving with distraction: an immersive fMRI study. Front. Hum. Neurosci. 7, (2013).
5. Uchiyama, Y., Ebe, K., Kozato, A., Okada, T. & Sadato, N. The neural substrates of driving at a safe distance: a functional MRI study. Neurosci. Lett. 352, 199–202 (2003).
6. Tsai, Y.-F., Viirre, E., Strychacz, C., Chase, B. & Jung, T.-P. Task performance and eye activity: predicting behavior relating to cognitive workload. Aviat. Space Environ. Med. 78, B176-185 (2007).
7. Salvucci, D. D. An interactive model-based environment for eye-movement protocol analysis and visualization. in Proceedings of the symposium on Eye tracking research & applications - ETRA ’00 57–63 (ACM Press, 2000). doi:10.1145/355017.355026.
8. Crundall, D. & Underwood, G. Visual Attention While Driving. in Handbook of Traffic Psychology 137–148 (Elsevier, 2011). doi:10.1016/B978-0-12-381984-0.10011-6.
9. Braga, R. M., Fu, R. Z., Seemungal, B. M., Wise, R. J. S. & Leech, R. Eye Movements during Auditory Attention Predict Individual Differences in Dorsal Attention Network Activity. Front. Hum. Neurosci. 10, (2016).
10. Hoeks, B. & Levelt, W. J. M. Pupillary dilation as a measure of attention: a quantitative system analysis. Behav. Res. Methods Instrum. Comput. 25, 16–26 (1993).
11. Kan, K., Schweizer, T. A., Tam, F. & Graham, S. J. Methodology for functional MRI of simulated driving: Functional MRI of simulated driving. Med. Phys. 40, 012301 (2012).
12. Cox, R. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. vol. 29 (Computers and Biomedical Research, 1996).
13. Talairach, J. & Tournoux, P. Co-planar stereotaxic atlas of the human brain: 3-Dimensional proportional system: An approach to cerebral imaging. (Thieme Medical Publishers, Inc.).
14. Shapiro, K., Hillstrom, A. P. & Husain, M. Control of Visuotemporal Attention by Inferior Parietal and Superior Temporal Cortex. Curr. Biol. 12, 1320–1325 (2002).
Figures