3935
Resting-State Functional Connectome Analysis of Awake Common Marmoset with Functional MRI and Electrocorticographic1Department of Radiological Sciences, Human Health Sciences, Tokyo Metropolitan University Graduate School, Tokyo, Japan, 2RIKEN Center for Brain Science, Saitama, Japan, 3Live Imaging Center, Central Institute for Experimental Animals, Kanagawa, Japan, 4Division of Regenerative Medicine, The Jikei University School of Medicine, Tokyo, Japan, 5Department of Physiology, Keio University School of Medicine, Tokyo, Japan, 6Graduate School of Computer Science and Systems Engineering, Kyushu Institute of Technology, Fukuoka, Japan, 7National Center of Neurology and Psychiatry, Tokyo, Japan
Synopsis
We explored the resting-state functional network of awake common marmoset by functional MRI (fMRI) and electrocorticographic (ECoG). As a result, a visual cortex network, a somatomotor network, a default mode network and a striatum network were detected in functional connectome analysis. In addition, some resting-state networks that were observed in the previous studies were detected in ICA. Those resting-state networks were also observed in ECoG data. Therefore, we conclude that fMRI and ECoG data were almost consistent.
Introduction
The connectome in brain neuroscience refers to each brain region and their interconnections.1 Recently, resting-state functional connectivity has been studied using various animal brains. Especially, resting-state functional connectivity of the common marmoset (Callithrix jacchus), which is a small New World primate, has been studied using functional magnetic resonance imaging (fMRI).2–4 fMRI reflects whole-brain correlations among the blood oxygen level dependent (BOLD) signals and has a great potential as a noninvasive method for investigating whole-brain circuitries. On the other hand, electrocorticography (ECoG), which is one of the methods for analyzing brain function, observes the electric field potential in the cerebral cortex and has high spatiotemporal resolution.5,6 The studies comparing fMRI and ECoG have been reported and the relationship between fMRI and ECoG is debatable. Here, we explored the resting-state functional network of common marmosets using fMRI and ECoG methods and compared the results by calculating fMRI and ECoG data.Methods
This study was conducted in the common marmoset (Callithrix jacchus), which has been used in recent neuroscience research.7-9 The functional magnetic resonance imaging (fMRI) protocol was applied to four healthy common marmosets (mean age, 4.90 ± 1.77 years; three males and one female). All imaging scans were performed using a 9.4-T MRI scanner (BioSpec 94/30; Bruker BioSpin, Ettlingen, Germany). Resting-state fMRI (true awake) was performed with the following parameters: gradient spin echo (EPI); TR/TE, 2,000/16 ms; repetition time, 150; isotropic resolution, 700 µm; one average; and acquisition time of one scan, 5 min (total acquisition time, 800 min). Functional magnetic resonance images were preprocessed (topup with FSL10,11 and realign, normalize and smoothing with spm12), and functional correlation coefficients between ROIs were generated (Figure 1). We collected resting-state electrocorticographic (ECoG) signals, which are comparable to local field potentials (LFPs) on the cortical surface, from three awake common marmosets. In this study, we use the 96ch ECoG array for common marmoset brain.5,6 This ECoG array covered almost the whole lateral surface of the hemisphere, from the occipital pole to the temporal and frontal poles, and provides an opportunity to capture global cortical information processing with high resolution at a sub-millisecond order in time and millimeter order in space.6Using resting-state fMRI and ECoG data, the functional connectivity was calculated. In addition, for each dataset from each subject, independent component analysis (ICA) was performed, and we explored the functional networks in a resting state. Several physiologically meaningful components were selected through visual inspection.
This study was approved by the Animal Experiment Committee at the RIKEN Center for Brain Science (CBS) in Japan and was conducted in accordance with the RIKEN CBS Guidelines for animal experiments.
Results and Discussion
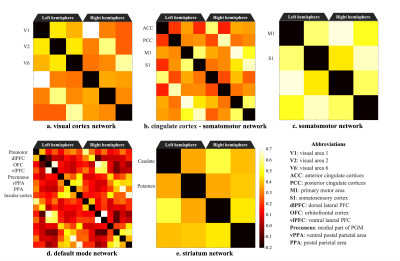
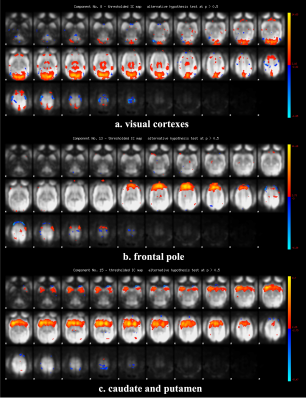
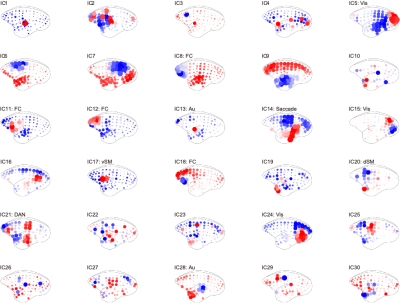
Figure 2 shows the functional connectivity matrix that was calculated using fMRI data. This result shows that the visual cortex network, somatomotor network, default mode network and striatum network matched the part of the network reported as the resting-state network (RSN) in the previous studies.3,4 Figure 3 shows the five RSNs observed in this study. Particularly, a somatomotor network (Figure 3-c) involving the primary motor area (M1) and the somatosensory cortex (S1) and a striatum network (Figure 3-e) involving caudate and putamen had strong functional connectivity. Furthermore, a strong functional connectivity was observed between a frontal pole (area 10) in left and right hemisphere. This region was identified as one of the RSN of awake marmoset in the previous study.3,4 Figure 4 shows the results of ICA using fMRI data. We found that some RSN was observed in subject ICA. Figure 4-a shows a network involving visual cortexes (IC8). Figure 4-b shows a striatum network involving a frontal pole (IC13). Figure 4-c shows a striatum network included caudate and putamen (IC15). Those networks were observed in awake marmoset in the previous studies.3,4 On the other hand, the resting-state networks on the raw ECoG included the visual, auditory, sensorimotor, dorsal attention, and frontal networks. Figure 5 shows the results of ICA using ECoG data. The RSNs on the raw ECoG included the visual, auditory, sensorimotor, dorsal attention, and frontal networks.12 Therefore, this finding may indicate that fMRI and ECoG were more or less consistent. This result is consistent with the previous studies showing that LFP is correlated with BOLD signals.13 However, some differences were observed in fMRI and ECoG data. It is possible that those differences were caused by the observation methods of brain function in fMRI and ECoG and differences in the spatiotemporal resolution. Our study has certain limitations, including a small number of subjects. Furthermore, in fMRI analysis, it is possible that functional connections related to the region at the base of the brain were affected by bad BOLD signal due to a magnetic susceptibility artifact effect.Conclusion
In this study, we explored the resting-state functional network of the common marmoset brain by fMRI and ECoG and compared fMRI and ECoG data. As a result, in fMRI and ECoG data, we detected some resting-state networks that were reported in the previous studies. This result suggests that fMRI and ECoG data were almost consistent. Additional studies are required to explore the resting-state network and understand brain function in a resting state.Acknowledgements
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI (JP19H04993, JP17H06034, MK; JP19H04998, JP17H06039, JP18KT0021, YY), JSPS CREST (JPMJCR16E2, YY), the program for Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from the Japan Agency for Medical Research and development (AMED) (JP19dm0207001, MK, TK, HO, TY, NI, YH, JH ; JP19dm0207069h0001, MK).
Conflict of Interest (COI) of the Principal Presenter:No potential COI to disclose
References
1. Sporns O, Tononi G, Kötter R. The human connectome: A structural description of the human brain. PLoS Computational Biology. 2005;1(4):0245–0251
2. Cirong Liu, Cecil Chern-Chyi Yen, Diego Szczupak, et al. Anatomical and functional investigation of the marmoset default mode network. Nature Communications. 2019;10:1975
3. Ghahremani M, Hutchison RM, Menon RS, et al. Frontoparietal Functional Connectivity in the Common Marmoset. Cereb Cortex. 2017;27(8):3890-3905
4. Belcher AM, Yen CC, Stepp H, et al. Large-scale brain networks in the awake, truly resting marmoset monkey. J Neurosci. 2013;33(42):16796-804
5. Komatsu M, Sugano E, Tomita H, et al. A Chronically Implantable Bidirectional Neural Interface for Non-human Primates. Front Neurosci. 2017;11:514
6. Komatsu M, Kaneko T, Okano H, et al. Chronic Implantation of Whole-cortical Electrocorticographic Array in the Common Marmoset. J Vis Exp. 2019;1(144)
7. Schilling KG, Gao Y, Christian M, et al. A Web-Based Atlas Combining MRI and Histology of the Squirrel Monkey Brain. Neuroinformatics. 2019;17(1):131-145
8. Hikishima K, Quallo MM, Komaki Y, et al. Population-averaged standard template brain atlas for the common marmoset (Callithrix jacchus). NeuroImage. 2011;54:2741–49
9. Okano H, Sasaki E, Yamamori T, et al. Brain/MINDS: A Japanese National Brain Project for Marmoset Neuroscience. Neuron. 2016;92(3):582-590
10. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870-888
11. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:208-219
12. Komatsu M, Yamada T, Kaneko T, et al. Resting
state networks on electrocorticograms reveal global and local cortical
functional structures. Society for Neuroscience. 2019;020.08
13. George A. Ojemann, Nick F. Ramsey and Jeffrey Ojemann. Relation between functional magnetic resonance imaging (fMRI) and single neuron, local field potential (LFP) and electrocorticography (ECoG) activity in human cortex. Neurosci. 2013;7:34
Figures
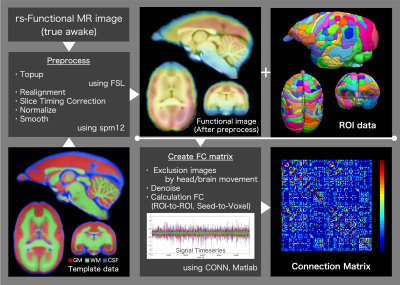
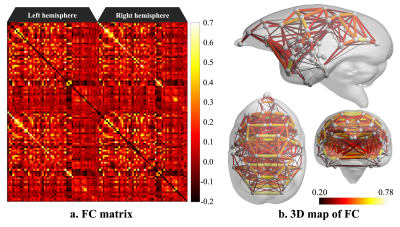
Figure 2. Functional connectivity matrix and 3D map of resting-state network in awake common marmoset