3931
Improved pre-surgical causal language mapping combining 3T and 7T fMRI with TMS and E-field modelling in a young tumour patient1Medical University of Vienna, Vienna, Austria
Synopsis
Here, we have optimised pre-surgical language mapping using navigated TMS with advanced E-field modelling and precise stimulation pulse timing. We employed a multi-modal approach incorporating ultra-high field (3 and 7 Tesla) functional magnetic resonance imaging, functional causal mapping including neuronavigated repetitive TMS, a software that allows for well-defined targeting and visual stimulus delay timings, as well as an advanced E-field/behavioural analysis. With this approach we could show that ‘virtual lesions’ close to tumour tissue yields clear spatial maps of functional impairment in language production.
Introduction
Pre-surgical planning is an essential part in the treatment for patients suffering from tumours or epilepsy, since it helps localising the actual surgical operation site1. FMRI has been proposed as a method for pre-surgical language mapping2. While fMRI, as a non-invasive mapping tool shows clear advantages over the intracarotid amobarbital procedure, fMRI results are limited by their pure correlational nature. Transcranial magnetic stimulation on the other hand allows for transient lesion-like effects at circumscribed cortical areas. Therefore, navigated repetitive TMS has been FDA approved for pre-surgical language mapping. However, it is characterised by inferior specificity compared to intra-operative stimulation mapping. Current applications face at least two challenges: (1) accurate triggering of TMS pulses based on the desired coil position and (2) exact estimation of the effective E-field distribution in the cortex. E-field modelling is superior to recent centre of the coil approaches for stimulation site definition, since it allows for a larger accuracy, in terms of estimated distribution of E-fields accounting for individual anatomy and information on the exact decline in field strength by cortical location and distance from peak area3.To address these issues we have developed a software that only triggers the visual stimulus and TMS-pulse if and only if the TMS coil is positioned within predefined error margins over a cortical target. Moreover, E-fields for each vector (position, orientation) of stimulation events were estimated to differentiate “effective” targets evoking speech arrest from ineffective targets. This reveals a causal functional map of eloquent cortical areas compared to pure centre of coil/function solutions in current procedures. By comparing this map with fMRI data using a paradigm developed for language localisation at 3 and 7 Tesla, we provide converging evidence for an optimised language mapping procedure.
Methods
Study Case. The study patient was a 32 year old man with a left-hemispheric insular tumour.Magnetic Resonance Imaging. A paradigm comprising auditory description decision previously shown to result in robust activations of language related areas4 was used. Two sessions were performed: (1) 7 Tesla EPI (Siemens MAGNETOM 7T, 32-channel head-coil, TR/TE=1400/23, voxel size = 1.5 x 1.5 x 1.5mm3, MB-factor=3); (2) 3 Tesla EPI (Siemens PRISMA, 64-channel head-coil, TR/TE=1000/35, voxel size =2.3 x 2.3 x 3mm3, MB-factor=4). The paradigm consisted of two conditions. In the test condition, the subject had to decide, if a sentence was semantically correct. In the control condition subjects had to listen to sentences played backwards and to detect a non-verbal sound at the end of the stimulus. High-resolution T1-weighted data was also acquired at both field strengths.
General Linear Model. Functional MRI data were realigned and smoothed with a 6 mm Gaussian kernel. Data analysis was performed using SPM12. Regressors were defined as “language forward” for test blocks and “language backwards” for control blocks. Data was then convolved with the canonical haemodynamic response function and linear contrasts “language forward”>”language backward” were calculated based on resulting β-estimates. T-statistics were set to p<.05, FWE corrected.
Transcranial magnetic stimulation. TMS was applied using a MagProX100 stimulator with an MRi-B91 MR-compatible TMS coil (Magventure, Farum, Denmark). For the definition of the motor threshold we stimulated the hand-knob of the left cerebral hemisphere based on anatomical information from the 3 Tesla anatomical image fed into the neuronavigation software. We decreased stimulation intensity until 3 out of 5 single-pulses led to a visually observable twitch in the first dorsal interosseus muscle. The active motor threshold was 62% of the maximal stimulator output. Trains of five pulses at 10Hz were applied at the respective targets of interest for language mapping as proposed in5,6.
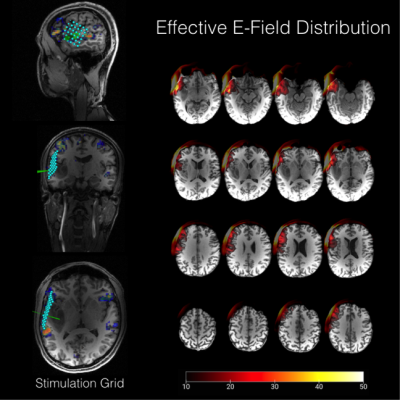
Trigger Software. Anatomical information of the head-model including the 6 x 6 target grid placed above the cortex covering the tumour (Figure 2) were then fed into our in-house built trigger software7. We additionally fed stimuli from the picture naming database into the software8. These stimuli were triggered 100 or 200 ms before the TMS train over the respective cortical targets. Each target was stimulated three times and the order of target positions was randomised.
E-field-modelling. For E-field modelling, location and intensity information from the TMS picture naming procedure was fed into the SimNIBS9 software in order to estimate E-field distribution for each target. Video recordings from the picture naming procedure were reviewed and evaluated based on qualitative (“speech arrest”) criteria. Subsequent differences of mean E-fields for “sure impairment” and “sure none-impairment” were calculated using FSL 5.0 (fslmaths).
Results
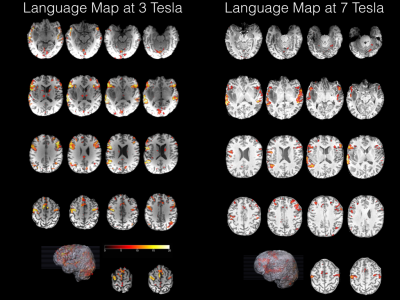
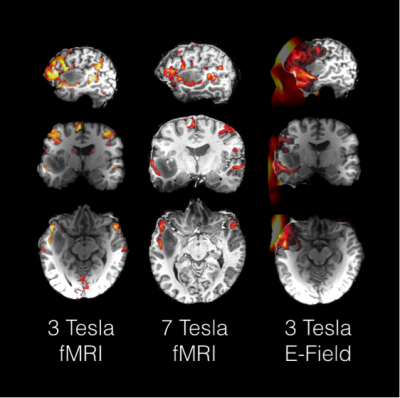
All runs led to statistically significant (p<.05 whole brain FWE corrected) activation increases in inferior frontal lobe, anterior midline alongside the superior temporal sulcus, anterior insula and inferior parietal lobules (Figure 1). Importantly, functional mapping results in the anterior perisylvian region in direct proximity to the tumour did strongly converge with causal mapping as revealed by TMS and subsequent E-field modelling (Figure 3).Discussion
Here, we propose an improved causal functional mapping procedure using E-field modelling for better spatial and a newly developed TMS trigger software for increased temporal control in pre-surgical language mapping.Conclusion
Technical advancements in comparison to standard language mapping protocols should be validated for clinical use in order to improve pre-surgical planning and improve functional outcomes.Acknowledgements
The authors would like to thank the FWF (Austrian Science Fund, P 33180-B) for financial support.References
1 Lefaucheur, J.-P. & Picht, T. The value of preoperative functional cortical mapping using navigated TMS. Neurophysiologie Clinique/Clinical Neurophysiology 46, 125-133 (2016).
2 Benjamin, C. F. et al. Presurgical language fMRI: mapping of six critical regions. Human brain mapping 38, 4239-4255 (2017).
3 Opitz, A., Windhoff, M., Heidemann, R. M., Turner, R. & Thielscher, A. How the brain tissue shapes the electric field induced by transcranial magnetic stimulation. Neuroimage 58, 849-859 (2011).
4 Berl, M. M. et al. Regional differences in the developmental trajectory of lateralization of the language network. Human brain mapping 35, 270-284 (2014).
5 Rösler, J. et al. Language mapping in healthy volunteers and brain tumor patients with a novel navigated TMS system: evidence of tumor-induced plasticity. Clinical Neurophysiology 125, 526-536 (2014).
6 Lioumis, P. et al. A novel approach for documenting naming errors induced by navigated transcranial magnetic stimulation. Journal of neuroscience methods 204, 349-354 (2012).
7 Fischer, R., Woletz, M., Tik, M. & Windischberger, C. A novel setup for high-precision neuronavigated TMS pulses. 25th Meeting of the Organization for Human Brain Mapping, HBM - Rome. (2019).
8 Szekely, A. et al. Timed action and object naming. Cortex 41, 7-25 (2005).
9 Saturnino, G., Antunes, A., Stelzer, J. & Thielscher, A. in 21st Annual Meeting of the Organization for Human Brain Mapping (OHBM 2015).
Figures