3927
Improving brain imaging in Parkinson's disease by accounting for simultaneous motor output.1Nuclear Magnetic Resonance, Max Planck Institute for Human Brain and Cognitive Sciences, Leipzig, Germany, 2Roche Pharma Research and Early Development, Roche Innovation Center Basel, Basel, Switzerland, 3Department of Neurology and Center of Clinical Neuroscience, , Charles University in Prague, Prague, Czech Republic, 4| CULS · Faculty of Environmental Science, Czech University of Life Sciences Prague, Prague, Czech Republic, 5Department of Environmental Engineering, Faculty of TechnologyTomas Bata University in Zlín, Zlin, Czech Republic, 6Neurology, General University Hospital in Prague, Prague, Czech Republic, 7Clinic for Cognitive Neurology, University Hospital Leipzig, Leipzig, Germany, 8Department of Neurology, Charles University in Prague | CUNI ·, Prague, Czech Republic
Synopsis
Parkinson's disease leads to a variety of movement impairments. While studying the disease with fMRI, the main motivation for the research becomes one of its major obstacles: the motor output is unpredictable. Therefore it is troublesome to access, inside the scanner, performances of motor tasks and reliably relate them to brain measurements. We proposed to overcome this by expanding the patients’ number and restricting statistical criteria from a previous study which used a glove with non-magnetic sensors during scanning. Our results revealed basal ganglia not observed in the previous study confirming the usefulness of the device in fMRI studies.
Introduction
In Parkinson's disease (PD), brain compensatory mechanisms typically begin before the patient acknowledges symptoms.1 Given that half the dopaminergic neurons are lost when subjects manifest motor alterations,2 a ‘silent’ adaptation results in changes from hyperactivity of globus pallidus (GP) to excitability increase of motor cortex. This imposes a challenge to imaging studies since both the motor corticostriato-thalamo-cortical loop3 (mcstcl) and the motor output are expected not to match. Consequently, the ability to accurately perform motor tasks during fMRI is impeded and deviations of the movement performance affect the results. Previous work has shown that kinematic modeling based on simultaneous measurements with MR-compatible gloves provides a means to address this problem.4 In the current work, we adopted this approach in a larger cohort along with conservative statistics employing family-wise error (FWE) correction at the voxel level to be less prone to produce false positives.Methods
Thirty-one right-handed PD patients performed 25 alternating sequences consisting of 10sec blocks of unilateral finger tapping and rest. Each patient underwent two scanning sessions in a 1.5T MAGNETOM Symphony scanner (Siemens, Erlangen, Germany) using a birdcage head coil and a gradient echo EPI sequence (TR=1000 ms, TE=54 ms, flip angle 90°). Ten coronal slices (thickness 3 mm, gap 1 mm, 3×3mm²) were obtained covering the basal ganglia and the primary motor cortex. The first session was performed, after a one-night withdrawal of L-dopa intake and the second session one hour after administration of 250 mg L-dopa/25 mg carbidopa (Isicom 250, Desitin Arzneimittel, Hamburg, Germany). Concomitantly with fMRI, patients used a non-magnetic glove device containing 14 fiber optic sensors (5th Dimension Technologies, Irvine, CA, USA) which captured a wide range of movement variability at a 64Hz sampling rate. Within and between subjects' movement differences were addressed by conducting, prior to the fMRI scan, a normalization procedure in which all patients were requested to perform calibration gestures to establish their individual peak amplitude and baseline values. fMRI data analysis was performed using SPM12 with Matlab R2017b. Pre-processing was performed with realignment for motion correction, normalization to the MNI standard space, and a Gaussian spatial filter of 10mm FWHM. Data sets were processed using a GLM including the glove data as a regressor.4 All session-specific sensor waveforms were averaged resulting in a single waveform (Figure 1). After parameter estimation, beta images were obtained for a second-level statistical analysis using a two-by-two factorial design [(L-dopa) on/off; (hand) left/right] as main effect of both factors. Significant results were obtained with p<0.05 at the voxel-level.Results
For both on and off conditions, a standard BOLD model on a second level analysis one-sample t-test revealed activation of the primary motor cortex contra-lateral to the tapping side. We further observed a ‘focusing effect’ related to L-Dopa intake5 as shown in Figure 2. With the glove regressor and for right-hand tapping, we further observed left putamen [−24, −2, 6], left caudate [−18, −10, 20] and left thalamus [−6, −10, 14] for the on condition (Figure 3).For the off condition, we also obtained left thalamus besides the motor areas. Comparing figures 3-4 we assume to observe an effect of l-Dopa in activating basal ganglia.
In order to assess the impact of L-dopa in the mcstcl, we performed a flexible factorial analysis using hand (left, right) and L-dopa (on, off) as factors, and computed a contrast by subtracting on minus off. For the standard model and right on condition, we observed exclusively the left thalamus. However, for the same condition using the glove regressor, we further obtained left posterior and right anterior putamen, thalamus and left GP (Figure 5).
Discussion
Our observation that sub-cortical structures like putamen, GP and thalamus emerge in brain imaging by use of the glove device leads us to interpret that the latter succeeds in better representing specific basal ganglia activations producing movement output. Furthermore, implicating the activation of posterior putamen and GP with the L-dopa intake, by means of the On–Off computation, corroborates with previous literature6,7,8 suggesting that the posterior putamen is typically the deteriorated structure that leads to PD. In the off state there is a lack of dopaminergic activation of putamen and GP8, whereas dopamine is delivered to the striatum due to L-dopa intake in the on state. The emergence of these nuclei is, hence, expected in response to a motor task as the neurotransmitter binds to the striatum’s D1 and D2 receptors.9 Although long-term chronic L-dopa treatment leads to D2 down-regulation,10 this hypothesis still holds as D2 receptors activate the indirect pathway (movement inhibitory) while D1 receptors activate the direct pathway (movement facilitation).Conclusion
Modeling the fMRI signal with the glove regressor resulted in substantial sensitivity improvement as compared to the standard model in line with previous work.4 Furthermore, due to our relatively large cohort, we could visualize activation in the basal ganglia at a greater level of detail. It is to note that these observations were obtained with a very conservative statistical approach. Taken together, our results suggest that kinematic modeling leads to a better understanding of the role of the basal ganglia in the study of movement in general and in investigations of treatment effects in PD patients in particular.Acknowledgements
No acknowledgement found.References
1. Lorraine V Kalia, Anthony E Lang, (2015) Parkinson's disease, The Lancet, Volume 386, Issue 9996, 2015, Pages 896-912, ISSN 0140-6736,
2. Fearnley, J. M., & Lees, A. J. (1990). Striatonigral degeneration: a clinicopathological study. Brain, 113(6), 1823-1842.
3. Alexander, G. E., DeLong, M. R., & Strick, P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual review of neuroscience, 9(1), 357-381.
4. Holiga, Š., Möller, H. E., Sieger, T., Schroeter, M. L., Jech, R., & Mueller, K. (2012). Accounting for movement increases sensitivity in detecting brain activity in Parkinson's disease. PLoS One, 7(5), e36271.
5. Ng, B., Palmer, S., Abugharbieh, R., & McKeown, M. J. (2010). Focusing effects of L dopa in Parkinson's disease. Human brain mapping, 31(1), 88-97.
6. Buchert, R., Lange, C., Spehl, T. S., Apostolova, I., Frings, L., Jonsson, C., ... & Hellwig, S. (2019). Diagnostic performance of the specific uptake size index for semi-quantitative analysis of I-123-FP-CIT SPECT: harmonized multi-center research setting versus typical clinical single-camera setting. EJNMMI research, 9(1), 37.
7. Redgrave, P., Rodriguez, M., Smith, Y., Rodriguez-Oroz, M. C., Lehericy, S., Bergman, H., ... & Obeso, J. A. (2010). Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nature Reviews Neuroscience, 11(11), 760.
8. Wurster, C. D., Graf, H., Ackermann, H., Groth, K., Kassubek, J., & Riecker, A. (2015). Neural correlates of rate-dependent finger-tapping in Parkinson’s disease. Brain Structure and Function,220(3), 1637-1648.
9. Lanciego, J. L., Luquin, N., & Obeso, J. A. (2012). Functional neuroanatomy of the basal ganglia. Cold Spring Harbor perspectives in medicine, 2(12), a009621.
10. Parenti, M., Flauto, C., Parati, E., Vescovi, A., & Groppetti, A. (1986). Differential effect of repeated treatment with L-DOPA on dopamine-D1 or-D2 receptors. Neuropharmacology, 25(3), 331-334.
Figures
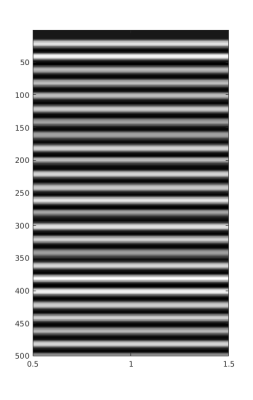
Figure 1.
Average of all 14 sensors waveforms after calibration, denoising, outliers correction and spectral inspection.
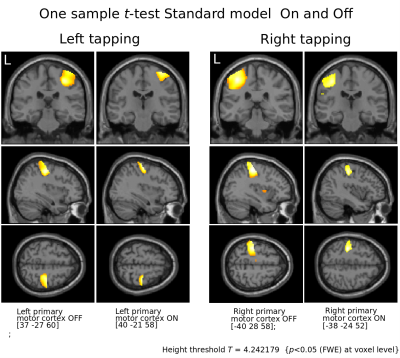
Figure 2.
Motor cortices contralateral to respective tapping.
Notice the focusing effect of L-Dopa suggested to be result from dopamine’s increase in signal to noise in cortical microcircuits.5
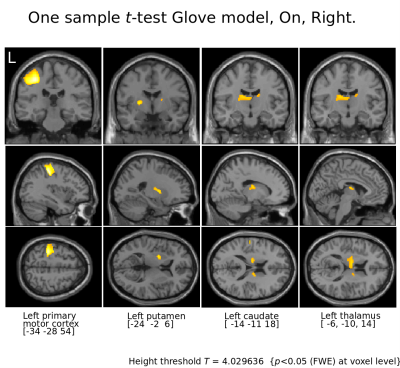
Figure 3.
Motor cortices and basal ganglia for Right tapping l-Dopa On.
Notice that with the Glove model not only the motor cortices are visible but also putamen, caudate and thalamus contralateral to the patient's tapping, all structures involved in the motor cortico-striato-thalamo-cortico loop.
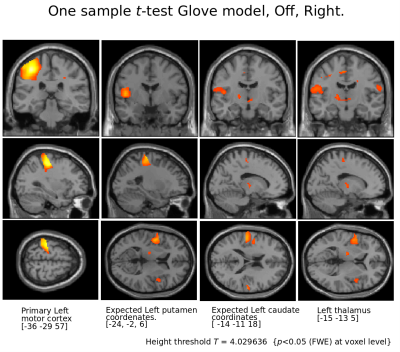
Figure 4.
Motor cortex and thalamus for Right tapping l-Dopa Off.
Notice that apart from motor cortex and thalamus, comparing brain coordinates expected to show putamen and caudate (as seen in On condition) no basal ganglia activity in absence of l-Dopa intake.
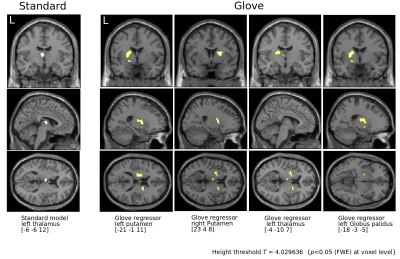
Flexible factorial analysis.
Condition On is subtracted from Off revealing basal ganglia activity from 31 PD patients under L-Dopa intake.