3923
Gold-Aluminum Composite Electrodes for Brain Stimulation and LFP Recording with Reduced Image Artifacts at 16.4 Tesla1Mechanical Engineering, University of Minnesota, Minneapolis, MN, United States, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
Understanding the mechanisms of deep brain stimulation (DBS) would benefit from the ability to perform DBS simultaneously with functional magnetic resonance imaging (fMRI), but DBS electrodes introduce severe artifacts in fMRI. We constructed a novel gold-aluminum composite wire bundle and tested it for reducing B0 field distortion and image artifacts in phantom and in vivo at ultrahigh field (UHF) of 16.4T. The composite wire bundle exhibited superior performance compared to controls made of only gold or only aluminum. The new composite material structure can be applied to make multi-channel DBS electrodes with significantly reduced image artifacts even in UHF fMRI.
Introduction
Deep brain stimulation (DBS) has the potential to treat a variety of neurological-based diseases and disorders, but its mechanisms are not fully understood 1–4. Combining DBS with functional magnetic resonance imaging (fMRI) introduces the possibility of mapping local and global brain responses and connectivity changes to focal DBS, but typical DBS electrodes introduce artifacts in MRI 5, 6 that are especially severe in gradient-echo (GE) echo-planar imaging (GE-EPI) sequences, and at high field strengths. The image artifacts are caused by a mismatch between the magnetic susceptibility of the foreign object and the surrounding tissue 7. Making surgical instruments from combined diamagnetic and paramagnetic material has been suggested to reduce image artifacts 7, for example by coating a paramagnetic guidewire with a diamagnetic material 8. In this abstract, we introduce a novel gold-aluminum composite structure that can be applied to make multi-channel DBS electrodes with substantially reduced image artifacts for animal research, even at the ultrahigh field (UHF) of 16.4T. The proposed electrode can be easily constructed without access to any specialized equipment.Methods
Gold and aluminum wires (100 micron and 125 micron diameters respectively) were stacked into groups of four, clamped at each end, twisted approximately 10 turns per centimeter, and trimmed. Three combinations were constructed: 4 gold wires, 4 aluminum wires, and a composite structure of 2 gold plus 2 aluminum wires.Wires were suspended in 2% wt. agarose phantoms and imaged with a single-loop proton RF surface coil in a 16.4T horizontal-bore research scanner. The wire bundle axis was arranged perpendicular to B0. B0 mapping was performed using a GE multi slice (GEMS) sequence with multiple echoes.
For comparison, analytical-based solutions of the B0 field map were computed for infinitely-long parallel cylinders perpendicular to B0 by superimposing the solution for a single cylinder which can be found in the literature 7.
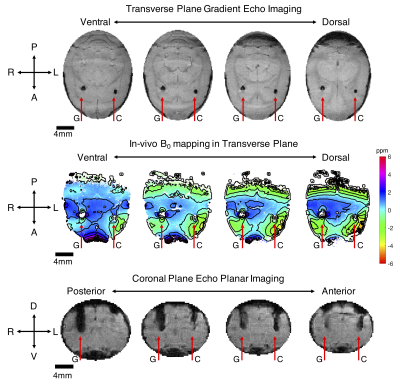
Additionally, a rat was bilaterally implanted with gold and composite wire bundles that were advanced into the subcortex. In vivo MRI was performed at 16.4T as described above. GEMS imaging and B0 mapping were performed, as well as multi-shot GE-EPI. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Minnesota.
Results
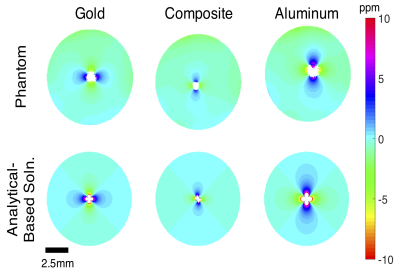
The experimental B0 field mapping revealed that the aluminum wire bundle produced the most field distortion (mapped in parts per million distortion in Fig. 1), followed by the gold bundle. The composite gold-aluminum bundle produced the least field distortion of the samples tested. The analytical-based solutions for each case matched the experimental results very well (Fig. 1).Results from the in vivo study at 16.4T agreed with the phantom study and computational results, and are presented in Fig. 2. The composite wire bundle created less signal loss in GEMS and GE-EPI imaging in comparison with the gold wire bundle. Additionally, the in vivo field map showed less field distortion around the composite wire bundle than the gold bundle.
Discussion
We have presented evidence that the gold-aluminum composite wire bundle produces less magnetic field distortion and smaller image artifacts in GEMS and GE-EPI imaging at 16.4T when compared to pure gold or pure aluminum bundle samples. Gold is a diamagnetic material with magnetic susceptibility of -34 ppm, whereas aluminum is paramagnetic with magnetic susceptibility of 20.7 ppm. The susceptibility of the tissue is assumed as -9 ppm 7. Assuming a linear volume mixing rule, the composite sample presented in this abstract has an effective magnetic susceptibility value of approximately 0.6 ppm. In comparison to traditional materials such as tungsten (77 ppm) and platinum (279 ppm) 7, all samples tested in this work are closer matched to the tissue susceptibility and therefore exhibit superior (imaging) performance.For the application of a multi-channel DBS electrode formed by a wire bundle, we are proposing to use a 1:1 ratio of gold and aluminum such that each wire has the same diameter and therefore the same tissue-contact area. The proposed 1:1 ratio has a theoretical magnetic susceptibility value of approximately -6.7 ppm (closer to brain tissue) and therefore should exhibit superior performance compared to the composite sample tested for the proof of concept in this work.
Conclusions and Future Work
The gold-aluminum composite wire structure showed superior performance due to better magnetic susceptibility matching to the surrounding media, specifically the brain tissue. Because magnetic field distortions are more problematic at higher magnetic field strengths (tested at 16.4T in this work), the composite electrode design should work as well or better at lower field strengths such as those used for clinical scanners (1.5T or 3T). In the future, we plan to fabricate the proposed DBS electrode design and test its utility and fidelity for brain stimulation and extracellular field-potential recording during fMRI acquisition in animal models. The proposed electrode structure is easy to reproduce and does not require any special equipment for fabrication and therefore could be applied broadly across the biomedical fields. One exciting opportunity is to apply the same concept for the next generation of high-density DBS for human patient application allowing MRI examination and tests after the DBS implantation.Acknowledgements
This work was supported in part by NIH grant R01 MH111413, S10 RR025031, NIBIB P41 EB027061, P30 NS076408, and by the University of Minnesota’s MnDRIVE (Minnesota’s Discovery, Research and Innovation Economy) initiative.References
1. McIntyre CC, Savasta M, Walter BL, and Vitek JL. How Does Deep Brain Stimulation Work? Present Understanding and Future Questions. J. Clin. Neurophysiol. 2004;21(1):40–50.
2. Johnson MD, Miocinovic S, McIntyre CC, and Vitek JL. Mechanisms and Targets of Deep Brain Stimulation in Movement Disorders. Neurotherapeutics. 2008;5(2):294–308.
3. Agnesi F, Johnson MD, and Vitek JL. Deep brain stimulation: how does it work? In Handbook of Clinical Neurology. 2013;116:39–54.
4. Chiken S and Nambu A. Mechanism of Deep Brain Stimulation: Inhibition, Excitation, or Disruption? Neuroscientist. 2016;22(3):313–322.
5. Pollo C, Villemure JG, Vingerhoets F, et al. Magnetic resonance artifact induced by the electrode Activa 3389: An in vitro and in vivo study. Acta Neurochir. 2004;146(2):161–164.
6. Lee JY, Kim JW, Lee JY, et al. Is MRI a reliable tool to locate the electrode after deep brain stimulation surgery? Comparison study of CT and MRI for the localization of electrodes after DBS. Acta Neurochir. 2010;152(12):2029–2036.
7. Schenck, JF. The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med. Phys. 1996;23(6):815–850.
8. Müller-Bierl B, Graf H, Steidle G, and Schick F. Compensation of magnetic field distortions from paramagnetic instruments by added diamagnetic material: Measurements and numerical simulations. Med. Phys. 2005;32(1):76–84.
Figures