3916
Insular subdivisions functional connectivity and immune dysregulation in patients with bipolar disorder: a resting‑state fMRI study1Medical Imaging Center, First Affiliated Hospital of Jinan University, Guangzhou, China, 2MR Research, GE Healthcare, Beijing, China
Synopsis
Systemic inflammation and immune dysregulation have been considered as risk factors in the pathophysiology of mood disorders including bipolar disorder (BD). Previous metabolism, structural and functional neuroimaging studies have reported the specific regional brain volumetric alteration and dysfunction of the insula in BD. Taken together, in current study, the associations between the whole-brain dFC of each insular subdivision in unmedicated patients with BD and the level of pro-inflammatory cytokines were analyzed. Our results demonstrated that the clinical risk factors might relate to the aberrant dFC in specific insular subdivision.
Introduction
Bipolar disorder (BD) is a chronic and recurrent disorder that has significant morbidity and mortality as they can lead to cognitive and functional impairment.1 Systemic inflammation and immune dysregulation have been considered as risk factors in the pathophysiology of mood disorders including BD.2-4 Resting-state functional magnetic resonance imaging (rs-fMRI) is a primary noninvasive neuroimaging technique that can reflects the changes of brain function status and resting state functional connectivity (rsFC) as a frequently used method, indicated by the temporal correlations in spatially separated brain regions, which may be clinically useful to the identification of BD. Previous metabolism, structural and functional neuroimaging studies have reported the specific regional brain volumetric alteration and dysfunction of the insula in BD. It can be identified 3 subregions including the anterior insula (AI), the middle insula (MI) and the posterior insula (PI). Each of them with distinct pattern of connectivity: an anterior region, functionally connects with frontal areas, anterior cingulate cortex and limbic areas, which is implicated in a wide range of conditions and behaviours, but also considered as a potential neural correlate of consciousness. A middle region and a posterior region, both primarily connected with sensorimotor areas, which are involved in sensorimotor processes. Thus far, studies of the immune system in psychiatric disorders have focused on classical markers of inflammation, such as interleukin (IL)-1β, IL-6, tumor necrosis factor-α (TNF-α) in serum (20-22), and Rosenblat et al also found that cognitive dysfunction was associated with elevated levels of pro-inflammatory markers IL-6, and TNF-α. In the current study, we collected rs-fMRI data and calculated the whole-brain FC of each insula subdivision in unmedicated patients with BD. We aim to investigate the relation between insular subdivisions FC and immune dysregulation in patients with bipolar disorder.Methods
Brain rs-fMRI data were acquired from 42 unmedicated BD II patients (current episode depressed), 69 healthy subjects. Three pairs of insula seed regions were selected: the bilateral AI [MNI (x, y, z): left = −32, 16, 6; right = 32, 16, 6], the bilateral MI (MNI: left = −38, 2, 8; right = 38, 2, 8), and the bilateral PI (MNI: left = −39, −15, 1; right = 39, −15, 8). And we examined the FC between the time series of the insula seed and the time series of other voxels. Levels of pro-inflammatory cytokines in serum, including IL-1β, IL-6, TNF-α, were determined from plasma in duplicate by high-sensitivity quantitative sandwich enzyme immunoassay kit (R& D Systems, Minneapolis, MN) run according to manufacturer’s directions. Finally, we calculated the correlation between the FC and the cytokines levels.Results and Discussion
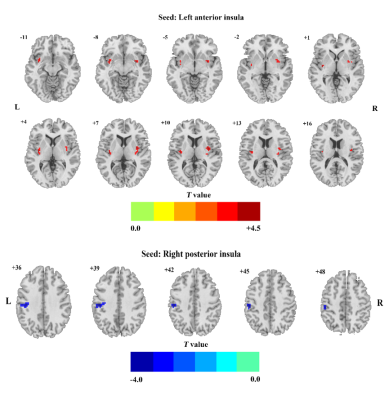
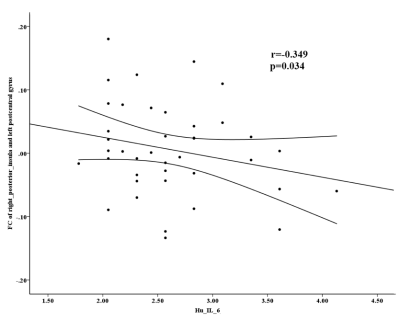
The main findings are the following: (i). Decreased FC between the right PI and left postcentral gyrus, and increased FC between the left AI and the bilateral insula (extended to the right putamen) in BD II depression (figure 1). (ii). Increased IL-6 and TNF-α levels in BD II depression than that in healthy controls (figure 1). (iii). Negative correlation between the abnormal FC and IL-6 levels in in the right posterior insula- left postcentral gyrus in BD II depression patients. As far as we know, this is the first study to assess the association between the FC and pro-inflammatory cytokines levels in unmedicated BD II depression, which may improve our understanding of BD from the perspective of the intrinsic brain activity and the immune system. The insula is part of the salience network (SN), which is the core neurocognitive networks identified in the human brain, played an important role in understanding higher cognitive function and affective processing in fundamental ways. The postcentral gyri are involved in primary somatosensory cortex, which is known to play a prominent role in the motor learning and processing of information. And the primary somatosensory cortex in the postcentral gyrus non-human primates contains neurons that encode roughness, applied force. Evidence supports a vital role for IL-6 in CNS physiology and pathology, which is as a regulator of pathways of neuronal and synaptic function. Our finding of increased FC between the left AI and the bilateral insula (extended to the right putamen) could be a compensatory response to structural deficits in BD, Findings of deficient FC between the PI and the motor area may be related to motor retardation of BD, and it may have correlation with immune dysregulation in BD to some extent.Conclusion
Our results showed that the BD II group exhibited decreased FC between the right PI and the left postcentral gyrus, and increased FC between the left AI and the bilateral insula (extended to the right putamen) when compared with HC group. For BD II depression patients, only the FC values in the right posterior insula- left postcentral gyrus showed a negatively correlation with IL-6 levels.Acknowledgements
No acknowledgement found.References
1. Vieta E, Berk M, Schulze TG, Carvalho AF, Suppes T, Calabrese JR, et al. Bipolar disorders. Nat Rev Dis Primers. 2018;4:16.
2. Boen E, Hjornevik T, Hummelen B, Elvsashagen T, Moberget T, Holtedahl JE, et al. Patterns of altered regional brain glucose metabolism in borderline personality disorder and bipolar II disorder. Acta psychiatrica Scandinavica. 2019;139(3):256-68.
3. Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS, et al. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and C-11 DASB; Comparison with bipolar disorder. Biological Psychiatry. 2007;62(8):870-7.
4. Yu H, Meng Y-j, Li X-j, Zhang C, Liang S, Li M-l, et al. Common and distinct patterns of grey matter alterations in borderline personality disorder and bipolar disorder: voxel-based meta-analysis. British Journal of Psychiatry. 2019;215(1):395-403.
Figures