3914
The amplitude of spontaneous fluctuations with high-frequency resting-state fMRI at 7T: influence of age and cardiorespiratory pulsations1Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 2School for Mental Health and Neuroscience, Maastricht University, Maastricht, Netherlands, 3Department of Electrical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands, 4Department of Neurology, Maastricht University Medical Center, Maastricht, Netherlands, 5Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, Netherlands, 6Maastricht Brain Imaging Center, Faculty of Psychology and Neuroscience, Maastricht University, Maastricht, Netherlands
Synopsis
Resting-state fMRI with a short TR enables unaliased sampling of the BOLD-signal, by disentangling cardiorespiratory pulsation signals. In this study, we aimed to explore the feasibility of obtaining a high-frequency spectrum and determined the influence of aging. Structural and high-frequency resting-state fMRI was performed using 7T MRI on 5 young and 5 elderly subjects. The power spectra, calculated for different brain regions, showed a clear separation of spontaneous BOLD fluctuations and the respiratory and cardiac pulsations. This pilot study demonstrated the feasibility of acquiring high-frequency spectra using fMRI. Furthermore, initial results confirm that the BOLD effect attenuates with aging.
Introduction
Resting-state (rs-)functional MRI is mostly used to detect spontaneous low-frequency fluctuations (10-100mHz). Dynamic scanning with a high sampling rate(>2Hz) enables unaliased sampling of physiological pulsations in the fMRI signal1,2. Therefore, it will be possible to discern the respiratory and cardiac signals from the fluctuations originating from the neuronal activity. In (cerebro)vascular disorders and ageing, these fluctuations may be affected and reflect abnormal neurovascular coupling due to vessel wall stiffening3,4. Using fMRI, previous studies found an increased cardiac pulsatility with aging in normal appearing white matter5,6. The first aim of this study was to explore the feasibility of obtaining an extended frequency spectrum in different brain regions, including the brainstem, using high-frequency rs-fMRI at 7T. The brainstem was also included, since this region is challenging for fMRI-measurements due to the pulsations of the circulating cerebrospinal fluid (CSF)7. Secondly, this technique was applied in a small cohort to study the influence of age on the amplitude of fluctuations.Methods
Subjects: 5 young healthy subjects (21-30 years,2 males) and 5 elderly healthy subjects (60-69 years,3 males).MRI acquisition: Images were acquired using a 7T MRI system (Magnetom, Siemens Healthineers, Erlangen, Germany) with a 32-channel phased-array head coil. To improve B1+ field homogeneity across the brain, dielectric pads were placed proximal to the temporal lobe. An MP2RAGE (TR/TE=5000/2.47ms,TI1/TI2=900/2750ms,α1/α2=5°/3°,cubic voxel size=0.7mm) was applied to acquire 3D whole-brain T1-weighted images for anatomical reference. The rs-fMRI time-series were acquired using a whole-brain multiband echo-planar-imaging sequence (TR/TE=383/17ms, multiband factor 4, pixel size 2.5x2.5mm2, sagittal slice thickness 2.5mm,48 slices,1000 volumes, duration 6:32min:sec). The short-TR achieves an effective sample frequency of 2.6Hz, obtaining a maximum frequency in the power spectrum of 1.3Hz, which enables unaliased sampling of the cardiac pulsations. Simultaneously, physiological signals were recorded using a pulse oximeter and a respiratory belt.
Image analysis: For preprocessing of the structural and the functional images, bias field correction, brain extraction and distortion correction were performed (FSL and SPM12). Furthermore, B1 correction8 was applied for the structural images, and these were segmented with FreeSurfer (v6.0.5)9, after which regions of interest (ROI) were defined in the brainstem and the gray matter of the superior frontal and temporal cortex. The latter two were chosen for their different proximity of large blood vessels. On the functional time-series, slice timing correction, deregression of white matter and CSF signal, and spatial alignment was performed. After preprocessing, the discrete power (Fourier) spectrum for the predefined ROIs was calculated. Furthermore, the amplitude of fluctuations (AF) was calculated in: the (standard) resting-state low frequency (10-100mHz), respiratory (200-400mHz) and cardiac range (750-1250mHz)10. These frequency ranges were defined based on the corresponding minimum and maximum frequency found in the spectra of the recorded physiological monitoring signals.
Statistics: For comparison of the AF between young and elderly subjects, a non-parametric Mann-Whitney U-test was performed (SPSS Statistics 25,IBM Inc.,Chicago,Illinois), with a significance level of 0.05.
Results
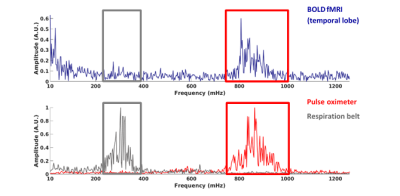
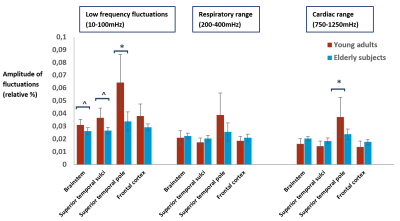
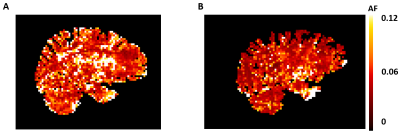
Figure 1 shows an example of the power spectrum of the BOLD-signal in the temporal cortex and the simultaneous recordings from the pulse oximeter and respiratory belt. The cardiac and respiratory peak can be observed in the BOLD-spectrum, and are supported by the physiological monitoring results. Comparison between young and elderly subjects reveal a significant lower AF in the low frequency range for the superior temporal cortex (pole) (p=0.016) for the elderly (Fig.2). Furthermore, a trend of lower AF in elderly subjects can be observed in the brainstem and temporal cortex for this frequency range (p=0.056). For the respiratory and cardiac frequency range, no significant differences were found between young and elderly subjects. However, it can be observed that the brainstem, superior temporal and frontal cortex appear to have a higher AF in the elderly compared to the young adults. The superior temporal pole shows a significantly higher AF in the young adults (p=0.016) compared to the elderly. Figure 3 depicts an example in the difference for the AF distribution for the low frequency range. The maps of the young adult show higher AF values than the maps of the older subject.Discussion
The multiband fMRI sequence with a short TR applied in this study was able to discern the respiratory and cardiac pulsations from the low frequency fluctuations originating from neurovascular coupling in all predefined brain regions. Significant trends in the low frequency range (10-100mHz) were found, where young adults had a higher amplitude for the fluctuations compared to the elderly subjects. To further confirm this observed effect, more subjects are currently being included. The different AF values found in the frontal and temporal cortex can be explained by the proximity and enhancement by freshly (i.e. unsaturated) inflowing spins from large vessels in these regions. Regions more closely located to the large vessels will experience more enhanced cardiac pulsations by blood flow.Conclusion
This study shows the feasibility to acquire unaliased high-frequency spectra in different brain regions, including the brainstem, using a whole-brain fMRI protocol with short TR (383ms). Initial results show that the native BOLD effect attenuates with aging. Future applications will be in hypertensive patients, which may gain more insights in the cerebral regulation of blood circulation and functional (cerebrovascular) abnormalities observed in hypertension.Acknowledgements
No acknowledgement found.References
1Trapp C, Vakamudi K, Posse S. On the Detection of High Frequency Correlations in Resting State fMRI. NeuroImage. 2018; 164: 202–213
2Tong Y, Hocke L, Frederick B. Short repetition time multiband echo-planar imaging with simultaneous pulse recording allows dynamic imaging of the cardiac pulsation signal. Magn. Reson. Med. 2014; 72(5): 1268–1276.
3O’Rourke M, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007; 50: 1–13
4Yang A, Thai S, Lin C, et al. Frequency and amplitude modulation of resting-state fMRI signals and their functional relevance in normal aging. Neurobiology of aging. 2018; 70: 59-69
5Makedonov I, Black S, MacIntosh B. BOLD fMRI in the White Matter as a Marker of Aging and Small Vessel Disease. PLoS ONE. 2013; 8(7): e67652
6Viessmann O, Möller H, Jezzard P. Dual regression physiological modeling of resting-state EPI power spectra: Effects of healthy aging. NeuroImage. 2019; 187:68-76
7Sclocco R, Beissner F, Bianciardi M, et al. Challenges and opportunities for brainstem neuroimaging with ultrahigh field MRI. Neuroimage. 2018;168: 412-426
8Marques J, Gruetter R. New Developments and Applications of the MP2RAGE Sequence - Focusing the Contrast and High Spatial Resolution R1 Mapping. PLoS One. 2013; 8(7): e69294
9Fischl B, Salat D, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002; 33(3):341-55
10Zou Q, Zhu C, Yang Y, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J. Neurosci. Methods. 2008; 172(1):137-41
Figures