3905
Negative correlations of brain activation between daily recognition and trauma memory remembering in PTSD
Kayako Matsuo1,2, Jun Inoue3, Toshiki Iwabuchi4, and Hidenori Yamasue5
1Center for Research Collaboration and Support, Dokkyo Medical University, Tochigi, Japan, 2Department of Psychiatry, Hamamatsu University School of Medicine (former), Hamamatsu, Japan, 3Department of Child and Adolescent Psychiatry, Hamamatsu University School of Medicine, Hamamatsu, Japan, 4Research Center for Child Mental Development, Hamamatsu University School of Medicine, Hamamatsu, Japan, 5Department of Psychiatry, Hamamatsu University School of Medicine, Hamamatsu, Japan
1Center for Research Collaboration and Support, Dokkyo Medical University, Tochigi, Japan, 2Department of Psychiatry, Hamamatsu University School of Medicine (former), Hamamatsu, Japan, 3Department of Child and Adolescent Psychiatry, Hamamatsu University School of Medicine, Hamamatsu, Japan, 4Research Center for Child Mental Development, Hamamatsu University School of Medicine, Hamamatsu, Japan, 5Department of Psychiatry, Hamamatsu University School of Medicine, Hamamatsu, Japan
Synopsis
We found a negative correlation between brain activity estimates of two conditions, daily recognition and trauma memory remembering, that reflected altered responses in PTSD. We conducted two task-fMRI runs for 9 patients with PTSD and the matched controls employing a script-driven imagery task. A region-of-interest analysis revealed a negative correlation between a hyperarousal subscale of psychological assessment and the activity estimate in the hippocampus in the daily recognition whereas a positive correlation in the trauma memory remembering. When computing voxel-based correlations between the activity estimates of the two conditions, extensive negative correlations emerged around the hippocampus in patients.
INTRODUCTION:
Posttraumatic stress disorder (PTSD) would alter brain responses and create a new pattern of activation. We aimed to characterize the altered pattern of activation in PTSD using fMRI. The second scan after a treatment of eye movement desensitization and reprocessing (EMDR) 1 was also conducted to observe how the treatment resolved the alteration. In a standard analysis, we typically apply a general linear model (GLM) to examine which task activates a brain location more extensively than other tasks. However, such a standard GLM method may have a disadvantage of failing to capture other types of features including a negative correlation between two tasks.METHODS:
Nine patients with PTSD (DSM-5 diagnosed) and nine age- and gender-matched controls (M/F=2/7) participated in the study after giving a written informed consent (approved by the institutional review board). Six out of the 9 patients also attended the second scan after the EMDR treatment (average 339.3 days of interval). A set of neuropsychological assessments including the Impact of Event Scale-Revised (IES-R-J) was conducted to patients. A 3-Tesla MRI was used to perform a BOLD fMRI with TR 2000 ms, TE 22 ms , flip angle 90 deg, FOV 192 mm, matrix 64 x 64, 45 axial-oblique slices, slice thickness 3 mm, interleaved, with no inter-slice space and 270 volumes. We adopted a script-driven imagery task 2 to conduct two runs of fMRI that presented two types of scripts respectively: patients’ own traumatic memory (Trauma task) and tooth-brushing movement as a daily activity (Tooth task). Both tasks contained blocks of the listening to a narration (Nar) and those of the remembering of it (Rem). After a standard preprocessing procedure, contrast estimates of Tooth Nar, Tooth Rem, Trauma Nar and Trauma Rem (against signals in blocks of a fixation) were computed individually using SPM12 (University College London, UK). We conducted a regression analysis to find locations whose activation was correlated to a score of the neuropsychological assessments and defined regions of interest (ROIs) with a diameter of 20 mm . We observed the correlations between the activity estimates in the ROIs and the subscale scores of the assessments.RESULTS:
The ROI analysis revealed a negative correlation between a hyperarousal subscale of the IES-R-J and activity estimates in the hippocampus in the Tooth Nar contrast, as well as a positive correlation between the same subscale and the estimates in the Trauma Rem (Fig. 1). This suggested that the estimates in the hippocampus would have negative correlations between the Tooth Nar and the Trauma Rem. To confirm this deduction, we computed correlations of activity estimates themselves by a voxel-based manner between the Tooth Nar and the Trauma Rem. We confirmed the negative correlation, which extended widely including the hippocampus, the amygdala, the insula and other temporal structures in patients (Fig. 2).DISCUSSION:
The brain responses of PTSD manifested in a way that patients who had an intense suppression of brain activation during daily recognition (Tooth Nar) showed an intense activation during trauma memory remembering (Trauma Rem), and vice versa. The EMDR apparently reduced the negative correlations (Fig. 2). The hippocampus was specifically remarked because it is a center of memory consolidation 3 and involved in altered cognition in PTSD 4. Actually, the involvement of the hippocampus in fMRI studies has been not necessarily clear and demonstrated a heterogeneity across studies 5-6. The heterogeneity might be explained by the negative correlation that we found because previous studies typically examined differences in the magnitude of activation between conditions and failed to detect the other types of relationships including negative correlation. Clinically, the negative correlation seems relevant to the “Windows of Tolerance” model 7-8. The “Window of Tolerance” refers to an optimum zone in which patients can efficiently function. Patients with PTSD often experience two extremes, hyperarousal (e.g., anger) and hypoarousal (e.g., dissociation), both of which are assumed to be outside the optimum zone 8. If the negative correlation reflected the alteration of these extremes, the magnitude of the flip might have a possibility for us to use it as an estimation of the patients’ responses to the treatment as well as the prognosis, which deserves a further investigation.CONCLUSIONS:
A feature of the brain response pattern in PTSD was found in the negative correlation of the activity estimates between the daily recognition and the trauma memory remembering. If a patient suppressed the activity in the hippocampus during the daily recognition, the activity during the trauma memory remembering would be enhanced, and vice versa. The negative correlation might have a relationship with frequent alterations of patients’ psychiatric states between hyperarousal and hypoarousal.Acknowledgements
This study was supported by the Grant-in-Aid for Challenging Exploratory Research (KAKENHI #15K13140) of the Japan Society for the Promotion of Science (JSPS).References
- Shapiro F. The role of eye movement desensitization and reprocessing (EMDR) therapy in medicine: addressing the psychological and physical symptoms stemming from adverse life experiences. Perm J. 2014;18(1):71-77.
- Lanius RA, Williamson PC, Boksman K, et al. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry. 2002;52(4):305-311.
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151-169.
- Fenster RJ, Lebois LAM, Ressler KJ, Suh J. Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat Rev Neurosci. 2018;19(9):535-551.
- Malejko K, Abler B, Plener PL, Straub J. Neural correlates of psychotherapeutic treatment of post-traumatic stress disorder: a systematic literature review. Front Psychiatry. 2017;8:85.
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann NY Acad Sci. 2006;1071:67-79.
- Ogden P, Minton K. Sensorimotor psychotherapy: one method for processing traumatic memory. Traumatology. 2000;3(3):149-173.
- Corrigan FM, Fisher JJ, Nutt DJ. Autonomic dysregulation and the Window of Tolerance model of the effects of complex emotional trauma. J Psychopharmacol. 2011;25(1):17-25.
Figures
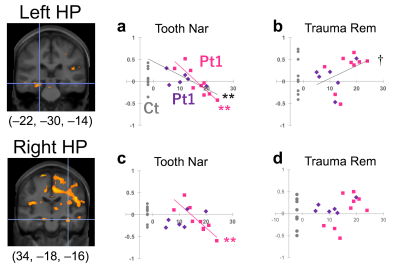
Figure 1. Correlations between a
hyperarousal subscale and activity estimates in the hippocampus (HP). Coronal
sections show centers of ROIs at the intersection of hairlines (MNI coordinates
below). Horizontal axis indicates the subscale score. Vertical axis indicates
activity estimates of the condition indicated at the header. Pt1, patients at
the first time. Pt2, patients at the second time. Ct, controls (no subscale). A
regression curve indicates a significant correlation. A pink line is for Pt1
whereas a dark gray, for Pt1 and Pt2 combined. **, p<0.01. †, p<0.10.
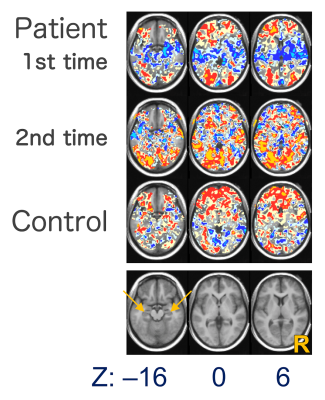
Figure 2. Voxel-based correlation maps between
activity estimates of Tooth Nar and Trauma Rem. The lowermost anatomical sections
indicate the corresponding axial slice levels to the maps at the z-coordinates
indicated below. The orange arrows indicate the hippocampi. R, right side of
the brain. Cyan color indicates the area whose correlation coefficients ≤ –0.8,
blue, ≤ –0.5, ice, ≤ –0.2, mustard, ≥ 0.8, red, ≥ 0.5 and cream, ≥ 0.2.