3895
Serum levels of inflammatory markers modulates brain structural changes and post-traumatic headache trajectory in mild traumatic brain injury1Department of Radiology, Washington University School of Medicine, St. Louis, MO 63110, St.Louis, MO, United States, 2Department of Medical Imaging, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China, Xi'an, China, 3The Key Laboratory of Biomedical Information Engineering, Ministry of Education, Department of Biomedical Engineering, School of Life Science and Technology, Xi’an Jiaotong University, Xi’an 710049, China, Xi'an, China
Synopsis
Post-traumatic headache (PTH) is one of the most frequent and persistent physical symptoms following mild traumatic brain injury (mTBI). However, the underlying neurobiological basis and modulatory component remained unclear. Evidence indicated that neuroinflammation is a major contributor in the pathogenesis of PTH. We hypothesized that the effect of peripheral inflammatory signaling on PTH could be produced by influencing brain structure that subserve pain modulatory function. Our findings demonstrated that neuroinflammation following mTBI is a potential process affecting structural changes in cognitive component of pain modulation, which may serve as a potential neurobiological mechanism underlying the emergence and persistence of PTH.
Introduction
Post-traumatic headache (PTH) is one of the most frequent and persistent physical symptoms following mild traumatic brain injury (mTBI). However, the underlying neurobiological basis and modulatory component remained unclear.1 Evidence indicated that neuroinflammation is a major contributor in the pathogenesis of PTH.2 We hypothesized that the effect of peripheral inflammatory signaling on PTH could be produced by influencing brain structure that subserve pain modulatory function.3 Peripheral inflammatory markers can interact with multiple central pathways as a principal channel for immune-to-brain communication in the development of pain states.2 This study aimed to test whether inflammatory processes affects PTH-specific grey matter volume (GMV) changes leading to pain dysfunction in early mTBI. Longitudinally, we also examined if these abnormalities can be persistent in patients developing chronic headache 3 months post-injury.Methods
77 mTBI patients initially underwent neuropsychological, peripheral blood inflammatory markers measurements and MRI scans within 7 days post-injury (T-1) and 54 (70.1%) of patients follow-up at 3-month (T-2). 42 matched healthy controls completed the same protocol at T-1 once. Voxel-based morphometry (VBM) analyses of GMV were conducted in PTH patients, compared with non-PTH patients and healthy volunteers at both initial and follow-up stages.Results
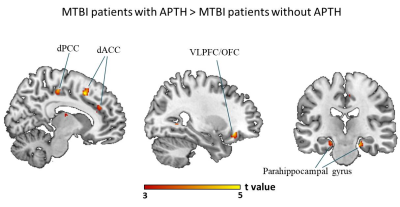
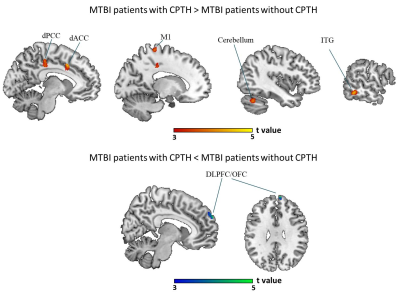
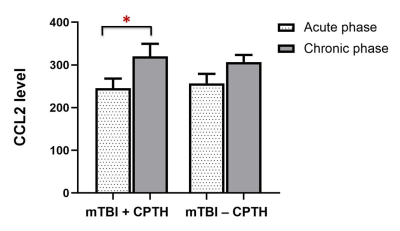
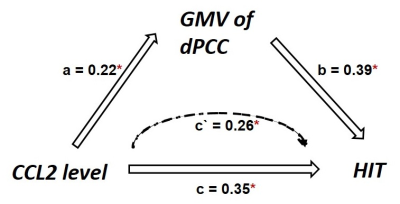
The mTBI patients with acute APTH presented significantly increased brain volume, mainly in the default mode network (DMN), compared with other two control groups. Among all the serum biomarker assay, stepwise regression analysis found that the initial C-C motif chemokine ligand 2 (CCL2) level significantly contributed to acute pain symptoms following mTBI, which was mediated by rapid greater GMV change within the key DMN region (dorsal posterior cingulate cortex, dPCC) in early mTBI. For the chronic stage, CCL2 level was significantly unregulated only in the mTBI patients with chronic PTH(CPTH),as well as GM volume increase in a subset of regions (dPCC and dACC) showing significant GMV group differences at the acute stage.Discussions and Conclusions
The increased GM changes were presented in the acute PTH, whereas bi-directional changes of GM were observed in the chronically persistent PTH. In the acute phase, the PCC structural change was shown to be an important mediator contributing the effect of CCL2 level on the dysfunctional pain symptoms observed in mTBI patients. Importantly, significant CCL2 rise over time and GM volume alterations in dPCC were found in patients developing CPTH. This study provide initial evidence that neuroinflammation following mTBI is a potential process4,5 affecting structural changes in cognitive component of pain modulation,6 which may serve as a potential neurobiological mechanism underlying the emergence and persistence of PTH in mTBI.7Acknowledgements
We thank all patients and healthy volunteers who participated in the study. This research was supported by the National Natural Science Foundation of China [Grant Numbers 81571752, 81771914].References
1. D. Nampiaparampil, Prevalence of chronic pain after traumatic brain injury: a systematic review, JAMA 300 (6) (2008) 711–719.
2.Mayer, C.L., Huber, B.R., and Peskind, E. (2013). Traumatic brain injury, neuroinflammation, and post-traumatic headaches. Headache 53, 1523–1530.
3. Niu X, Bai L. Disruption of periaqueductal grey-default mode network functional connectivity predicts persistent post-traumatic headache in mild traumatic brain injury. 2019;90(3):326-32. doi: 10.1136/jnnp-2018-318886
4. Illias AM, Gist AC, Zhang H, et al. Chemokine CCL2 and its receptor CCR2 in the dorsal root ganglion contribute to oxaliplatin-induced mechanical hypersensitivity. Pain 2018;159(7):1308-16. doi: 10.1097/j.pain.0000000000001212
5. Zhu X, Cao S, Zhu MD, et al. Contribution of chemokine CCL2/CCR2 signaling in the dorsal root ganglion and spinal cord to the maintenance of neuropathic pain in a rat model of lumbar disc herniation. The journal of pain : official journal of the American Pain Society 2014;15(5):516-26. doi: 10.1016/j.jpain.2014.01.492
6. Obermann M, Nebel K, Schumann C, et al. Gray matter changes related to chronic posttraumatic headache. Neurology 2009;73(12):978-83.
7. Alshelh Z, Marciszewski KK, Akhter R, et al. Disruption of default mode network dynamics in acute and chronic pain states. NeuroImage Clinical 2018;17:222-31.
Figures