3893
Aberrant amplitude of low frequency fluctuations of spontaneous brain activity in patients with Parkinson’s disease
Yanjun Liu1, Mengyan Li2, Xinhua Wei3, Xiuhang Ruan3, Guihe Hu2, Haobo Chen2, and Yaoqin Xie1
1Institute of Biomedical and Health Engineering, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2Department of Neurology, Guangzhou First People’s Hospital, School of Medicine, South China University of Technology, Guangzhou, China, 3Department of Radiology, Guangzhou First People’s Hospital, School of Medicine, South China University of Technology, Guangzhou, China
1Institute of Biomedical and Health Engineering, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2Department of Neurology, Guangzhou First People’s Hospital, School of Medicine, South China University of Technology, Guangzhou, China, 3Department of Radiology, Guangzhou First People’s Hospital, School of Medicine, South China University of Technology, Guangzhou, China
Synopsis
Parkinson’s disease (PD) patients are widely reported with abnormalities in motor and cognitive features. Early diagnosis can be benefit if neuroimaging markers are well developed. This study investigated the altered amplitude of low frequency fluctuations (ALFF) of brain activity by functional MRI and explored the neural correlates of motor and cognitive symptoms of PD. Compared to normal controls, PD patients exhibited increased ALFF in default mode network and decreased in cerebellum. The ALFF was negatively correlated with the motor performances of PD in cerebellum. The findings suggest the cerebellum as critical area associated with motor and cognitive performances in PD.
INTRODUCTION
Patients with Parkinson’s disease (PD) are widely reported with abnormalities in motor and cognitive features, which have an impact on the overall quality of life for the affected people 1. New neuroimaging technologies may contribute in the early diagnosis of PD if neuroimaging markers are well developed. A great number of neuroimaging researches have been focus on it, while the exact underlying mechanisms are complicated and remain unclear. This study investigated the alterations of low frequency fluctuations of spontaneous brain activities and explored the neural correlations of motor and cognitive functions in PD patients.METHODS
Age- and gender-matched of 35 normal controls (NC) and 36 PD patients and were enrolled in the resting-state MRI scanning. All participants were required to lie quietly during MRI scanning and stayed awake with eye closed. The resting functional image and structural T1 image were obtained from all participants. In addition, the clinical assessments including motor and non-motor symptoms were measured across all PD patients. The motor symptoms were identified by the motor part of Unified Parkinson’s Disease Rating Scale (UPDRS-III) 2. The non-motor symptoms were assessed by evaluating the cognitive functions, including the Frontal Assessment Battery (FAB) associated with frontal function 3, and the mini-mental state examination (MMSE) 4. The neuroimaging data preprocessed were performed by DPABI 5, including time points removal, slice timing, head-motion correction, segmentation by DARTEL 6, covariates regression, spatial normalization to MNI space, linear detrending, smoothness with FWHM of 4 mm, and filtering with band frequency pass of 0.01-0.1Hz. The amplitude of low frequency fluctuations (ALFF) 7 was employed to measure the fluctuations of spontaneous brain activity. To evaluate between-group differences, two sample t-test was performed on the standardized ALFF (zALFF) maps of NC and PD, corrected by Gaussian Random Field (GRF) with voxel p < 0.005 and cluster p < 0.05 (t > 2.91, cluster size >1296mm2) within gray matter mask, with gender, age and head motion of FD Jenkinson as covariates. The brain regions showing significant ALFF differences were extracted as regions of interests (ROI) and the ALFF signals were extracted and then correlated with the clinical assessments to investigate the neural correlations on motor and cognitive functions in regarding to PD.RESULTS
Compare with NC, PD patients were observed with increased ALFF in the left para-hippocampus (extending to olfactory cortex) and left angular gyrus (AG) extending to middle temporal gyrus (MTG) (Figure 1, Figure 2A). While it was decreased in the right precentral gyrus (PreC), right inferior temporal gyrus (ITG), left cerebellum anterior lobe (CAL) and right cerebellum posterior lobe (CPL) (Figure 1, Figure 2A). The PD group exhibited significantly decreased MMSE rating scores (p = 0.0004) (Figure 2B) and FAB scores (p = 0.0015) than NC (Figure 2C). The correlative analysis of ALFF and clinical assessments within the significant brain regions was demonstrated in Figure 3. The ALFF of NC group was negatively correlated with MMSE scores in the left ITG (r =-0.34), negatively correlated with MMSE scores in the right CPL(r =-0.49), and positively correlated with FAB scores in the left CAL (r = 0.36), while all of them were decoupled (p > 0.05) for PD patients (Figure 3A,3B,3C). The neural correlates of motor performance of PD showed negative correlations between ALFF and UPDRS-III scores in the right PreC and right CPL (Figure 3D).DISCUSSION
PD is a kind of neurodegenerative disease characterized by motor impairments and sometimes accompanied with cognitive deficits. ALFF of PD was observed increased in para-hippocampus and posterior AG/MTG and decreased in precentral gyrus and cerebellum. Hippocampus is well known as its role in cognition and memory, whereas it’s reported to be associated with motor functions as well 8. Posterior AG/MTG are critical hubs of default mode network in which activations are observed during task-deprived state and deactivations during task-related state 9. The cerebellum and precentral gyrus is associated with both sensorimotor and cognitive processing 10. Aberrant brain activity in default mode network and sensorimotor area indicate abnormalities of cognitive and motor functions in PD. PD were shown negative correlations between ALFF and motor scores in precentral gyrus and posterior cerebellum, suggesting the decreased motor function of PD is related to aberrant brain activity in sensorimotor area.CONCLUSION
This study provides new insights on the interaction among the low frequency fluctuations of brain activity, motor and non-motor functions in PD. The findings suggest that cerebellum is critical area that associated with motor and cognitive functions of PD, which may be potential neuroimaging markers in identifying PD.Acknowledgements
This work is supported in part by grants from Shenzhen matching project (GJHS20170314155751703), National Key R&D Program of China (2016YFC0105102), National Science Foundation of China (61871374, 81871846), Leading Talent of Special Support Project in Guangdong (2016TX03R139), Science and Technology Planning Project of Guangzhou (201804010032), Guangzhou Municipal Health Bureau Project (20171A010247), Guangzhou Key Project of R&D Innovation (2016201604030018), Fundamental Research Program of Shenzhen (JCYJ20170413162458312), and Shenzhen Engineering Laboratory for Key Technologies on Intervention Diagnosis and Treatment Integration.References
- Kudlicka, A., Clare, L. & Hindle, J. V. Executive functions in Parkinson’s disease: Systematic review and meta-analysis. Movement Disorders 26, 2305–2315 (2011).
- Goetz, C. G. et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 (2008).
- Dubois, B., Slachevsky, A., Litvan, I. & Pillon, B. The FAB: A frontal assessment battery at bedside. Neurology 55, 1621–1628 (2000).
- Folstein, M. F., Folstein, S. E. & McHugh, P. R. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198 (1975).
- Yan, C. G., Wang, X. Di, Zuo, X. N. & Zang, Y. F. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 14, 339–351 (2016).
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113 (2007).
- Zang, Y. F. et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 29, 83–91 (2007).
- Li, M. et al. Altered Global Synchronizations in Patients With Parkinson’s Disease: A Resting-State fMRI Study. Front. Aging Neurosci. 11, (2019).
- Raichle, M. E. et al. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 98, 676–682 (2001).
- Kansal, K. et al. Structural cerebellar correlates of cognitive and motor dysfunctions in cerebellar degeneration. Brain 140, 707–720 (2017).
Figures
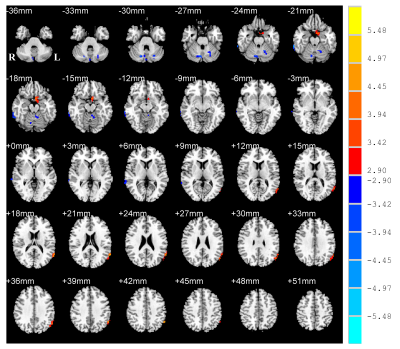
Figure
1. Statistical T-map of ALFF differences between NC (normal controls) and Parkinson’s
disease (PD). Multiple comparison corrections are implemented by Gaussian
Random Field (GRF) with voxel p <
0.005 and cluster p<0.05 (t > 2.91, cluster size >1296mm2)
within gray matter mask. The color bar is the statistical t-value. Red/blue
overlays indicate higher/lower ALFF in PD. ALFF, amplitude of low frequency
fluctuations; L/R, left/right hemisphere.
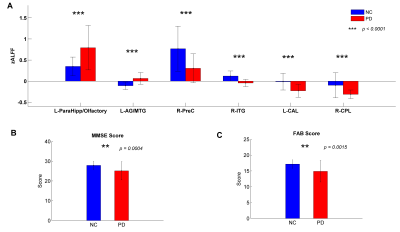
Figure
2. (A) ALFF signals of two groups
extracted from the brain regions of significant between-group differences. (B) MMSE scores of normal controls
(NC) and Parkinson’s disease (PD). (C)
FAB scores of NC and PD. ALFF, amplitude of low frequency fluctuations; L/R,
left/right; ParaHippo, para-hippocampus; AG, angular gyrus; MTG/ITG,
middle/inferior temporal gyrus; PreC, precentral gyrus; CAL/CPL, cerebellum
anterior/posterior lobe.
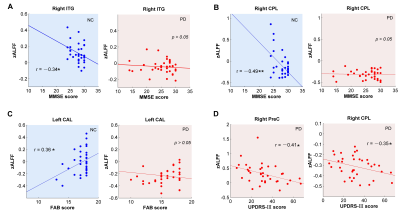
Figure
3. Correlations between brain activity (ALFF) and cognitive/motor functions (MMSE/
FAB/UPDRS-III) within brain regions of significant between-group differences.
Significance notations: * p<0.05,
** p<0.005. NC, normal controls;
PD, Parkinson’s disease; MMSE, mini-mental state examination; FAB, frontal assessment
battery; UPDRS-III, motor part of Unified Parkinson’s Disease Rating Scale;
ITG, inferior temporal gyrus; PreC, precentral gyrus; CAL/CPL, cerebellum
anterior/posterior lobe.