3883
Parallel transmit (pTx) with online pulse design for task-based fMRI at 7T1Wolfson Brain Imaging Centre, University of Cambridge, Cambridge, United Kingdom, 2MRC Cognition and Brain Science Unit, Cambridge, United Kingdom, 3Siemens Healthcare Limited, Erlangen, Germany, 4Siemens Healthcare Limited, Firmley, United Kingdom
Synopsis
Parallel transmit (pTx) has developed in recent years to show promising reductions in signal dropouts and imaging artifacts from B1+ field inhomogeneities in 7T MRI. However, pTx methods have rarely been applied for functional MRI. To our knowledge, no published task fMRI study has used pTx with online pulse calculation. We therefore implemented pTx spokes excitation in our vendor’s product EPI sequence, and tested it in volunteers using two different task-based fMRI paradigms. Comparing with CP+ (or “TrueForm”) mode, using pTx spokes pulses significantly improved both the tSNR and fCNR in task-based fMRI acquisitions at 7T.
Introduction
At ultra-high fields(≥7T), the B1+ fields generated by single channel coils are typically rather inhomogeneous in the human head, resulting in signal dropouts in some brain regions. Parallel transmit (pTx) techniques are able to improve image homogeneity and overall quality at 7T1. Previous 7T pTx-fMRI acquisitions have been largely limited to resting state data2,3. Here, we compare the performance of a pTx excitation pulse against sinc excitation in the circularly polarised (CP+, or “TrueForm”) RF shim for an fMRI study with two different task fMRI paradigms.Methods
pTx sequence: The vendor’s standard gradient echo EPI sequence (ep2d) was modified to use the pTx pulse design framework (Siemens). The imaging slab (30 slices) was divided into 5 groups and spokes-2 pulses were designed using the framework on the 3rd (i.e. centre) slice in each group. Control scans used a circularly polarised (CP+) RF shim and sinc excitation. Image acquisition: 6 subjects (3 females, age=28.3±4.5years) were scanned with an 8Tx32Rx head coil (Nova Medical, USA) on a Magnetom Terra 7T system (Siemens, VE11U/K software). Acquisition parameters for all EPI scans were: 30 axial slices across the temporal lobe, FA=40°, TR/TE=2500/25ms, resolution=2.0×2.0×2.3mm3(slice direction), GRAPPA=2(24 reference lines), readout bandwidth=1748Hz/Px, phase-encode=A>P. A scan with the same parameters as above but with reversed phase gradient polarity(5 volumes) and a whole-brain structural image(MP2RAGE, 0.75mm3 isotropic resolution) were also acquired.Visual stimuli and task: fMRI datasets were acquired during two different visual paradigms with pTx-EPI and CP+-EPI. Paradigm 1 consisted of a visual stimulus involving images of faces (F), objects (O), scenes (S) and patterns (P) in 16s blocks (25 images/block). 16 image blocks (4 each) were interleaved with 4 blocks of rest (16s), giving a total time of around 6 minutes per run. In each block, some images were repeated and subjects were required to detect immediate repetitions by pressing a response key (one-back task). Paradigm 2 consisted of a semantic association (Se) and a pattern matching task (Pa). In the semantic association task, three pictures were presented for each trial - a probe picture on the top and two pictures at the bottom. The subject was required to select the picture that was more related in meaning to the probe. The pattern-matching task had an identical layout but the subject now had to choose the pattern identical to the probe. Each run (about 9 minutes) consisted of 11 alternating blocks of each task (16s, 4 trials) with rest blocks (10s) between them. In addition, one extra resting state dataset was acquired for each sequence (100 volumes).
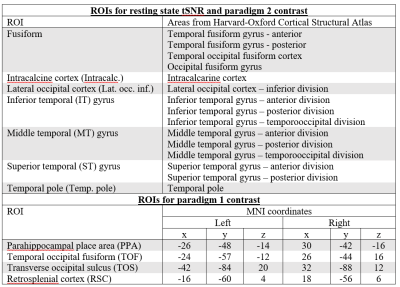
Data processing: Data were analysed using FSL (v6.0.1). All EPI runs were distortion corrected with topup, then motion corrected with MCFLIRT before further analysis. From the resting state data, tSNR maps were obtained by taking the mean of the time series divided by its standard deviation, before registering to the standard MNI152 brain. Percentage tSNR change [δtSNR=(tSNRpTx-tSNRCP)/(tSNRpTx+tSNRCP)*200%] was calculated in 7 ROIs(Table 1)4. For task data, each time-series was smoothed with a Gaussian kernel (FWHM=4mm). The BOLD response for each run was modelled by convolving the corresponding explanatory variables(4 for paradigm 1 and 2 for paradigm 2) with a hemodynamic response function. General Linear Model analyses that included motion parameters as regressors were performed. For paradigm 1, the following contrasts were computed: S>F, S>O, with a threshold of p<10-4 (uncorrected); while for paradigm 2, the Se>Pa contrast was computed with threshold of clusters determined by z>3.1 and a corrected significance cluster threshold of p<0.05. Functional contrast-to-noise ratio (fCNR=amplitude/standard deviation of noise) and number of active voxels (nVoxels) were calculated for various ROIs and their percentage changes (δfCNR and δnVoxels) are defined similarly to δtSNR. ROIs for paradigm 1 were based on previous literature data5,6 (Table 1) while the same 7 ROIs used for tSNR calculations were used for paradigm 2.
Results
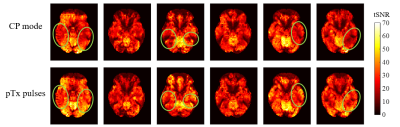
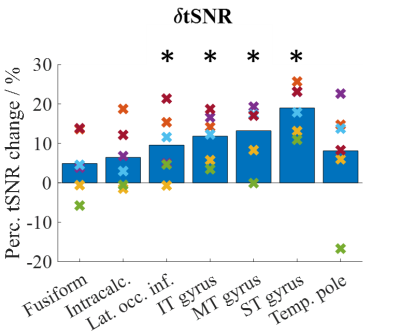
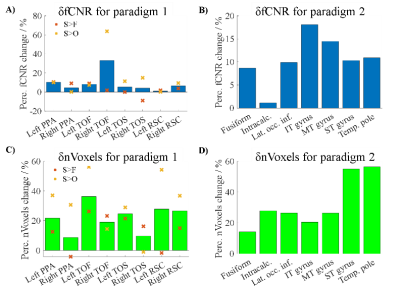
tSNR: Figure 1 shows the tSNR map from a transverse slice in each volunteer. CP+ acquisition displayed signal loss in the lower temporal lobe region (green circles) which was recovered by using pTx excitation. Figure 2 shows the δtSNR across the 7 ROIs for all volunteers. On average across all subjects, the pTx-mode outperformed the CP+ mode in terms of tSNR in all 7 ROIs, with statistically significant increases (Student’s paired t-test) in: lat. occ. inf., IT, MT and ST gyrus. The average increase across all ROIs was 10.4%.Functional data: With both paradigms, CP+-EPI and pTx-EPI scans showed significant activation in the temporal and fusiform regions as expected5–7. Figure 3 shows the subject-average δfCNR and δnVoxels for paradigm 1 and 2. Larger cluster sizes and increased fCNR were observed with the pTx-mode compared to CP+ in the regions of interest.
Discussion & Conclusions
The results from this experiment show the potential value of spokes-2 pTx excitation for task-based fMRI. Compared to the standard sinc CP+ excitation approach (CP+-EPI), pTx-EPI was able to increase tSNR across the whole brain and reduce signal dropouts in the temporal lobes. In addition, with simple targeted task-based fMRI, we showed that the pTx pulse design enabled an average of 9.5% increase in fCNR and 26.1% increase in cluster size in brain regions related to place-object perception and visual association.Acknowledgements
BD is supported by Gates Cambridge Trust. CR is funded by the NIHR Cambridge Biomedical Research Centre and the Isaac Newton Trust. CTR is funded by a Sir Henry Dale Fellowship from the Wellcome Trust and the Royal Society [098436/Z/12/B]. This study was funded by the NIHR Cambridge Biomedical Research Centre and MRC Clinical Research Infrastructure Award for 7T.References
1. Padormo, F., Beqiri, A., Hajnal, J. V. & Malik, S. J. Parallel transmission for ultrahigh-field imaging. NMR Biomed. 29, 1145–1161 (2016).
2. Wu, X. et al. Human Connectome Project-style resting-state functional MRI at 7 Tesla using radiofrequency parallel transmission. Neuroimage 184, 396–408 (2019).
3. Gras, V., Poser, B. A., Wu, X., Tomi-Tricot, R. & Boulant, N. Optimizing BOLD sensitivity in the 7T Human Connectome Project resting-state fMRI protocol using plug-and-play parallel transmission. Neuroimage (2019). doi:10.1016/J.NEUROIMAGE.2019.03.040
4. Rua, C. et al. Improving fMRI in signal drop-out regions at 7 T by using tailored radio-frequency pulses: application to the ventral occipito-temporal cortex. Magn. Reson. Mater. Physics, Biol. Med. 31, 257–267 (2018).
5. Watson, D. M., Hartley, T. & Andrews, T. J. Patterns of response to visual scenes are linked to the low-level properties of the image. Neuroimage 99, 402–410 (2014).
6. Hodgetts, C. J., Shine, J. P., Lawrence, A. D., Downing, P. E. & Graham, K. S. Evidencing a place for the hippocampus within the core scene processing network. Hum. Brain Mapp. 37, 3779–3794 (2016).
7. Jung, J., Williams, S. R., Sanaei Nezhad, F. & Lambon Ralph, M. A. GABA concentrations in the anterior temporal lobe predict human semantic processing. Sci. Rep. 7, 15748 (2017).
Figures