3881
Segmented spin-echo BOLD fMRI using a variable flip angle FLEET acquisition with recursive RF pulse design for high spatial resolution fMRI1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Vanderbilt University Institute of Imaging Science, Nashville, TN, United States, 4Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
Spin-echo (SE) EPI has long been desired for fMRI acquisitions with reduced macrovascular sensitivity; however, to achieve a purely T2-weighted signal, requires sampling a short window around the spin-echo—generally achieved by a segmented readout. Segmented EPI is well-known to be temporally unstable due to sensitivity to subject motion between segments. Here we propose a reordering of the EPI segments, known as FLEET, combined with a variable flip angle excitation to maximize the image signal level and a recursive RF pulse design to maintain consistent slice profiles at the spin-echo. We demonstrate the feasibility of this approach at 3T.
Introduction
Blood oxygenation level-dependent (BOLD) functional MRI (fMRI) using a spin-echo (SE) acquisition has long been pursued due to its reduced macrovascular sensitivity as compared to gradient-echo (GE) BOLD,1–3 making SE-BOLD more spatially specific to neuronal activation at ultra-high field.4,5 In practice, however, SE BOLD fMRI uses an EPI readout, which has R2' decay away from the spin-echo time, resulting in substantial macrovascular sensitivity.6,7 Undersampling along the phase-encode direction will shorten the readout window, however impractical levels of acceleration are required to achieve high imaging resolution. Using a segmented EPI readout can reduce the R2'-weighting 7 but this makes the acquisition vulnerable to subject motion between segments (~2s inter-segment TR), resulting in intermittent ghosting artifacts and reduced image quality. It was recently shown that the temporal stability of segmented GE EPI can be markedly improved with a FLEET (Fast Low Excitation-angle Echo-planar Train) readout that acquires all shots for a given slice prior to acquiring the next slice’s data.8 To ensure optimal SNR and consistent signal levels across shots, a variable flip angle (VFA) acquisition with recursively designed Shinnar-Le Roux (SLR) RF pulses 9 that ensured consistent slice profiles across shots was used. Here, we have combined the recursive pulse design with a spin-echo VFA-FLEET acquisition to produce a segmented-accelerated SE-EPI acquisition (SE-VFA-FLEET) with reduced motion-vulnerability.Theory
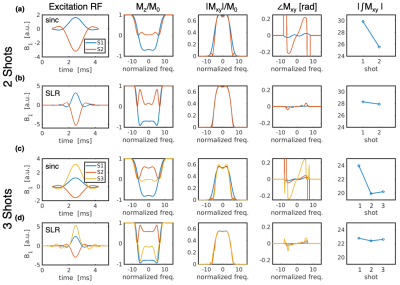
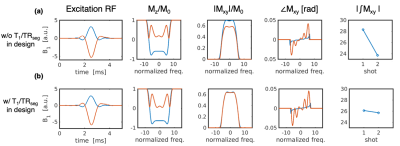
Target flip angles in the SE-VFA-FLEET scheme were determined recursively as in standard GE-VFA:10 $$\alpha_{i-1}=\tan^{-1}(\sin(\alpha_i)).$$ To maximize magnetization, the final excitation was set to 90°, giving $$$\alpha_i$$$={45°,90°} or {35°,45°,90°} for 2- or 3-shots, respectively (with no dummies). The recursive SLR pulse design generates a standard excitation-refocusing pulse pair for the first shot then accounts for attenuation of $$$M_z$$$ to design the subsequent excitation pulses while matching the $$$M_{xy}$$$ profile from the first shot. Scaling a standard Hann-windowed sinc RF pulse to the desired flip angles results in non-uniform slice profiles from shot-to-shot since the $$$M_z$$$ profile changes prior to each excitation (Figure 1). To facilitate consistent excitation slice profiles across shots, the refocusing pulse was designed to be 1.4x wider than the excitation profile. Due to the accelerated relaxation experienced after inversion to the –z-axis, and the non-negligible time between shots (~100 ms) expected for SE-VFA-FLEET, the ratio of T1 to the segment TR ($$$TR_{seg}$$$) was factored into the pulse design (Figure 2).Methods
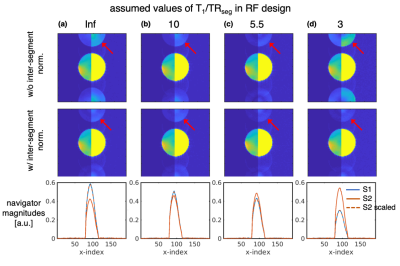
All experiments were conducted at 3T. To examine the impact of T1 relaxation on shot-to-shot signal consistency, a homogeneous agar phantom with a short T1 (550 ms) was scanned using a 12-channel receive head coil at low-resolution with a large field-of-view to resolve the ghosts. Acquisition parameters were: 2 shots, 96×96 matrix, 4.4-mm in-plane resolution, 3.1-mm slice thickness, 15 slices, 50% slice gap, TE=60ms, $$$TR_{seg}$$$=100ms, volume TR ($$$TR_{vol}$$$)=3s. Scans were repeated using sets of pulses that were designed with $$$T_1/TR_{seg}=\{\infty,10,5.5,3\}$$$ with the expectation that the $$$T_1/TR_{seg}$$$=5.5 pulses would provide the best results given the $$$TR_{seg}$$$ and T1 of the phantom.A healthy volunteer was scanned at 3T using a 32-channel receive coil. A range of $$$T_1/TR_{seg}$$$ was used in the pulse design and testing to account for the wide range of T1 in brain tissue. Unaccelerated and combined segmented-accelerated acquisitions were acquired—in each case performing 10 repetitions to assess image quality, including stable and unstable ghosts.
Images were reconstructed offline using the FID navigators to perform within segment Nyquist ghost correction. To account for differences in shot-to-shot signal, a scaling factor that minimized the mean-square error between navigators was applied across segments. Scaling factors were determined from a separately acquired calibration scan identical to the imaging scan but with no phase-encoding gradients. GRAPPA reconstruction with FLEET-ACS 11 was applied to the combined accelerated segments.
Results
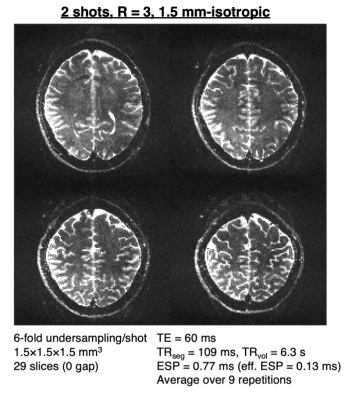
Figures 2 and 3 demonstrate the non-negligible impact of T1 relaxation in the RF pulse design. As predicted, in the agar phantom, the $$$T_1/TR_{seg}$$$=5.5 pulses resulted in the lowest level of ghosting in the phantom. Remarkably, ghosting levels were reduced to near comparable levels by the inter-segment normalization in all sets of pulses.In the in vivo scan, the image quality from the pulses designed with $$$T_1/TR_{seg}$$$=7 and 10 were qualitatively best—ghosting signal typically originated from the periphery. Figure 4 shows an example single repetition using 3-shots and the impact of inter-segment normalization on ghost reduction. Figure 5 shows a high-resolution 1.5-mm isotropic acquisition achieved with 2-shots R=3 and averaged across 9 repetitions (first repetition discarded as a dummy).
Discussion and Conclusions
We have presented a novel pulse sequence to acquire segmented SE-EPI with reduced vulnerability to subject motion. Using the recursive RF pulse design, we could achieve a SE-VFA-FLEET acquisition that incorporates inversion of $$$M_z$$$ during refocusing and relaxation across segments while achieving consistent slice profiles across shots. Due to the multiple pulses used, significant gradient spoiling and crushing were required, however, further optimization of these gradient moments will be investigated to improve signal stability and reduce ghosting. Ghosting from tissues whose T1 did not match the T1 used in pulse design was problematic but could be addressed, possibly, by using more segments, each of shorter duration. In future work, we aim to implement the sequence at 7T where SE-EPI will have greater value for high-resolution fMRI.Acknowledgements
This work was supported in part by the CIHR (MFE–154755), the NIH NIBIB (grants P41-EB015896, R01-EB019437, and R01-EB016695), by the BRAIN Initiative (NIH NIMH grant R01-MH111419 and NIBIB grant U01-EB025162), and by the MGH/HST Athinoula A. Martinos Center for Biomedical Imaging; and was made possible by the resources provided by NIH Shared Instrumentation Grants S10-RR023043 and S10-RR019371.References
1. Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM. MR Contrast Due to Intravascular Magnetic-Susceptibility Perturbations. Magn Reson Med 1995;34:555–566.
2. Norris DG. Spin-echo fMRI: The poor relation? Neuroimage 2012;62:1109–1115 doi: 10.1016/j.neuroimage.2012.01.003.
3. Koopmans PJ, Yacoub E. Strategies and prospects for cortical depth dependent T2 and T2* weighted BOLD fMRI studies. Neuroimage 2019;197:668–676 doi: 10.1016/j.neuroimage.2019.03.024.
4. Yacoub E, Duong TQ, Van de Moortele PF, et al. Spin-echo fMRI in humans using high spatial resolutions and high magnetic fields. Magn Reson Med 2003;49:655–664 doi: 10.1002/mrm.10433.
5. Duong TQ, Yacoub E, Adriany G, Hu XP, Ugurbil K, Kim SG. Microvascular BOLD contribution at 4 and 7 T in the human brain: Gradient-echo and spin-echo fMRI with suppression of blood effects. Magn Reson Med 2003;49:1019–1027 doi: Doi 10.1002/Mrm.10472.
6. Birn R, Bandettini PA. The effect of T2' changes on spin-echo EPI-derived brain activation maps. Proc Intl Soc Mag Reson Med. 2002;10:1324.
7. Goense JB, Logothetis NK. Laminar specificity in monkey V1 using high-resolution SE-fMRI. Magn Reson Imaging 2006;24:381–392 doi: 10.1016/j.mri.2005.12.032.
8. Berman AJL, Witzel T, Grissom WAG, Park D, Setsompop K, Polimeni JR. High-resolution segmented-accelerated EPI using Variable Flip Angle FLEET with tailored slice profiles. In: International Society of Magnetic Resonance in Medicine Annual Meeting. Vol. 27. Montreal, Canada; 2019. p. 1169.
9. Pauly J, Leroux P, Nishimura D, Macovski A. Parameter Relations for the Shinnar-Leroux Selective Excitation Pulse Design Algorithm. IEEE Trans Med Imaging 1991;10:53–65 doi: Doi 10.1109/42.75611.
10. Mansfield P. Spatial mapping of the chemical shift in NMR. Magn Reson Med 1984;1:370–386.
11. Polimeni JR, Bhat H, Witzel T, et al. Reducing sensitivity losses due to respiration and motion in accelerated echo planar imaging by reordering the autocalibration data acquisition. Magn Reson Med 2016;75:665–679 doi: 10.1002/mrm.25628.
Figures